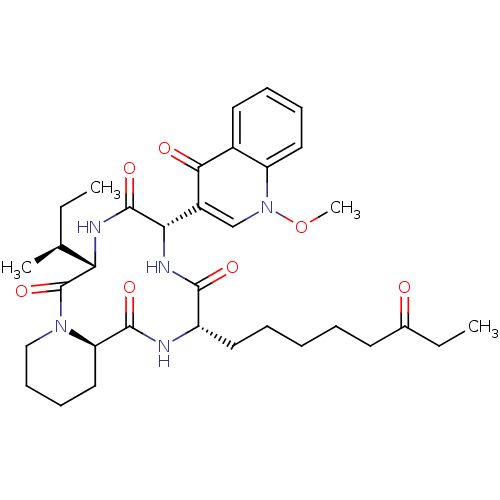
BDBM50366733 CHEMBL1793973
SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O)c1cn(OC)c2ccccc2c1=O
InChI Key InChIKey=IEVZGTIIJVLPRE-YVHAWAGASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50366733
Found 4 hits for monomerid = 50366733
Affinity DataKi: 30nMAssay Description:Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoaMore data for this Ligand-Target Pair
Affinity DataIC50: 86nMAssay Description:Inhibitory activity against histone deacetylase (HDAC) enzyme derived from partially purified extracts of human HeLa cells using [3H]11 as radioligan...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Tested for Histone deacetylase enzyme inhibition assay using Eimeria tenella extractMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against histone deacetylase enzyme derived from partially purified extracts of Eimeria tenella protozoa using [3H]11 as radioliga...More data for this Ligand-Target Pair