BDBM50396262 CHEMBL2172322
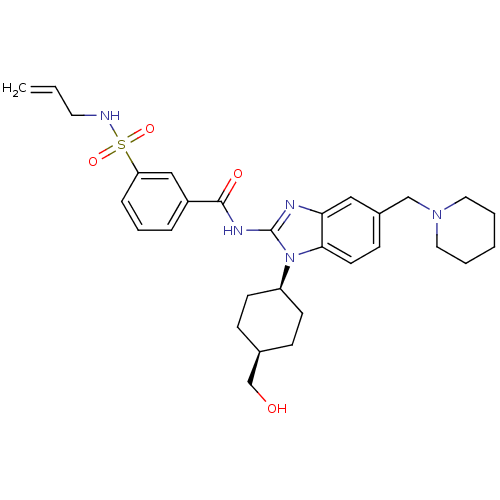
SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)S(=O)(=O)NCC=C)nc2cc(CN3CCCCC3)ccc12
InChI Key InChIKey=LWJAHJMKAARBCO-WZJNIGMMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50396262
Found 3 hits for monomerid = 50396262
Affinity DataIC50: 48nMAssay Description:Inhibition of ALK Tyr1604 phosphorylation by cell based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.92E+3nMAssay Description:Inhibition of c-Met enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of ALK enzymeMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)