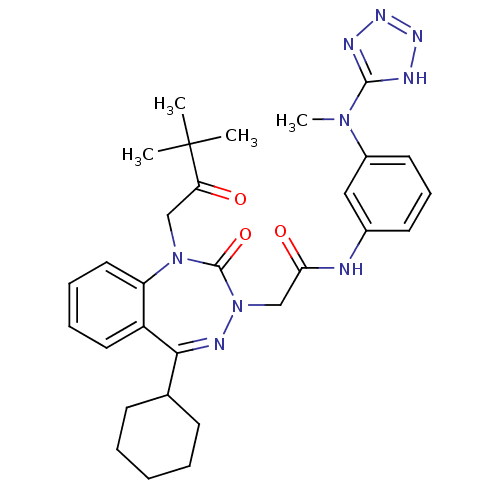
BDBM50411338 CHEMBL227254
SMILES CN(c1nnn[nH]1)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1
InChI Key InChIKey=FYQXEGHPESKWGJ-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50411338
Found 3 hits for monomerid = 50411338
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
James Black Foundation
Curated by ChEMBL
James Black Foundation
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cellsChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosaChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 6.46E+3nMAssay Description:Displacement of [3H]L-364718 from human recombinant CCK1 receptor expressed in PC3 cellsChecked by AuthorMore data for this Ligand-Target Pair