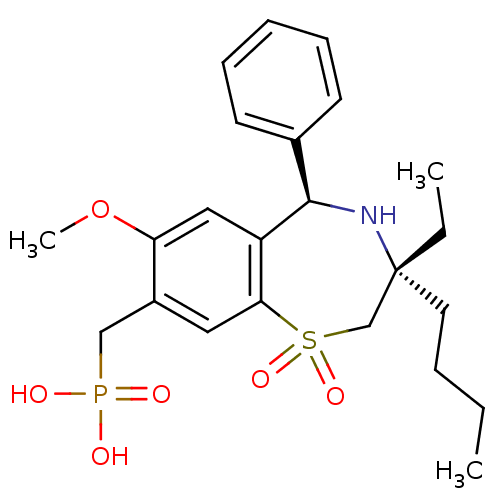
BDBM50434849 CHEMBL2385105::US9040518, 6
SMILES CCCC[C@]1(CC)CS(=O)(=O)c2cc(CP(O)(O)=O)c(OC)cc2[C@H](N1)c1ccccc1
InChI Key InChIKey=KSROWRDKNMGWAM-DHIUTWEWSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50434849
Found 4 hits for monomerid = 50434849
Affinity DataIC50: 23nMT: 2°CAssay Description:On the day of the uptake experiment, 10 mM HEPES was added to Hank's Balanced Salt Solution, and the pH was adjusted to 7.4 with TRIS (HBSSH). The as...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysisMore data for this Ligand-Target Pair