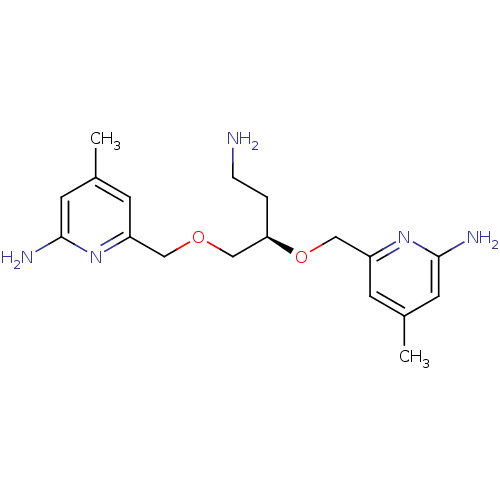
BDBM50441014 CHEMBL2430147::US9732037, Compound 17
SMILES Cc1cc(N)nc(COC[C@@H](CCN)OCc2cc(C)cc(N)n2)c1
InChI Key InChIKey=BXQXCGJXAWOXHN-MRXNPFEDSA-N
Data 6 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50441014
Found 6 hits for monomerid = 50441014
Affinity DataKi: 881nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli using L-arginine as substrate up to 180 seconds by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 881nMAssay Description:NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre...More data for this Ligand-Target Pair
Affinity DataKi: 3.14E+4nMAssay Description:NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
Northwestern University
Curated by ChEMBL
Northwestern University
Curated by ChEMBL
Affinity DataKi: 3.14E+4nMAssay Description:Inhibition of mouse recombinant iNOS expressed in Escherichia coli using L-arginine as substrate up to 180 seconds by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.70E+4nMAssay Description:NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre...More data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
Northwestern University
Curated by ChEMBL
Northwestern University
Curated by ChEMBL
Affinity DataKi: 6.70E+4nMAssay Description:Inhibition of bovine recombinant eNOS expressed in Escherichia coli using L-arginine as substrate up to 180 seconds by hemoglobin capture assayMore data for this Ligand-Target Pair