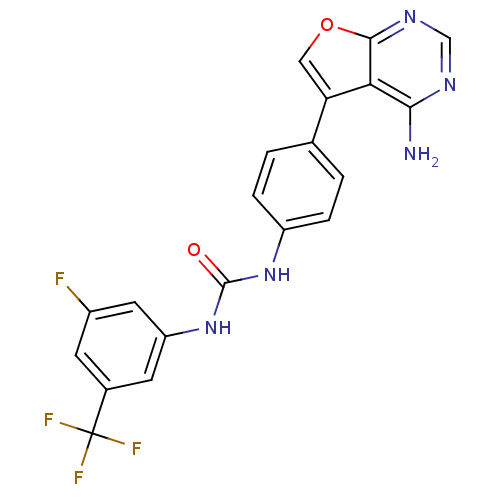
BDBM50446262 CHEMBL3109216
SMILES Nc1ncnc2occ(-c3ccc(NC(=O)Nc4cc(F)cc(c4)C(F)(F)F)cc3)c12
InChI Key InChIKey=BASRWXZKOTYWGV-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50446262
Found 3 hits for monomerid = 50446262
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Displacement of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of human N-terminal His-GST-TEV-fused RIP1 kinase domain (1 to 375) autophosphorylation expressed in baculovirus infected insect Sf9 cells...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of RIP1 in human U937 cells assessed as prevention of TNFalpha/zVAD.fmk-induced necrotic cell death preincubated for 30 to 60 mins followe...More data for this Ligand-Target Pair