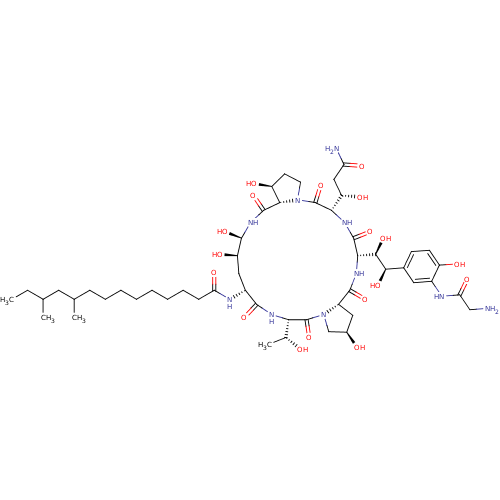
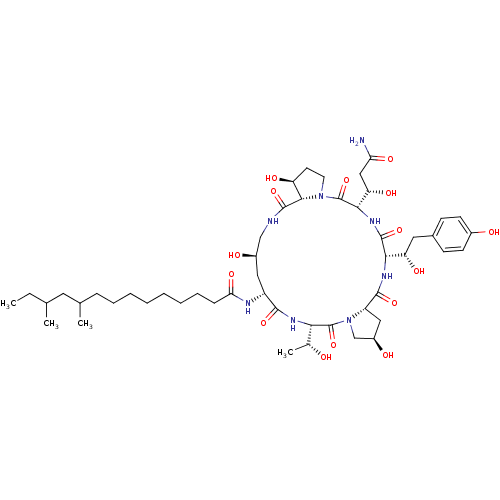
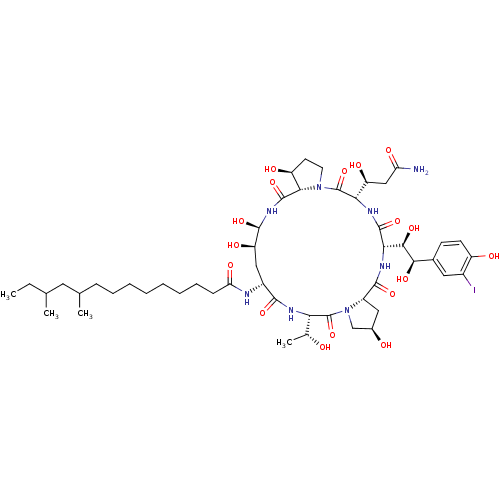
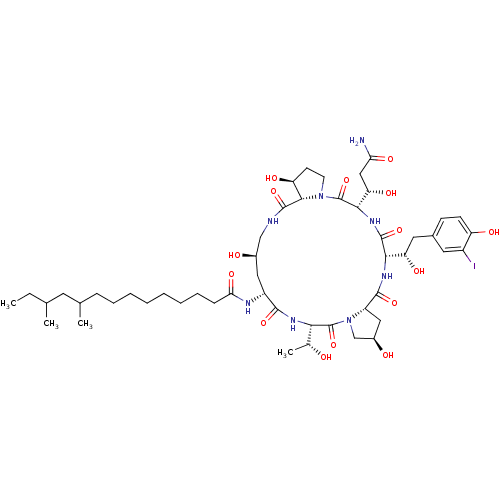
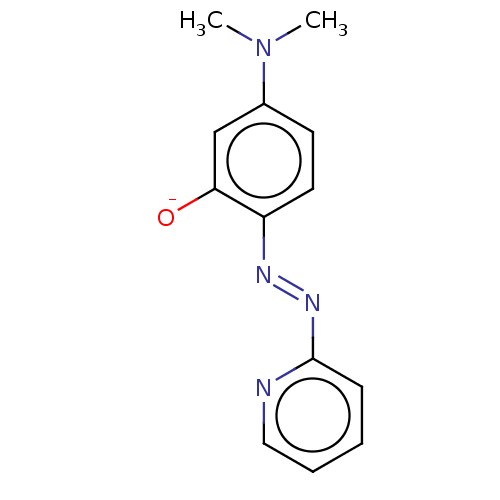
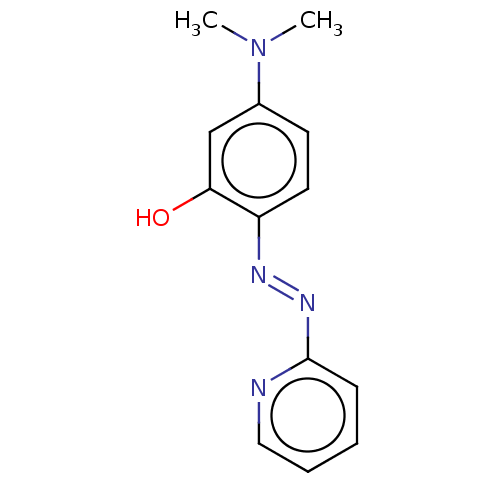
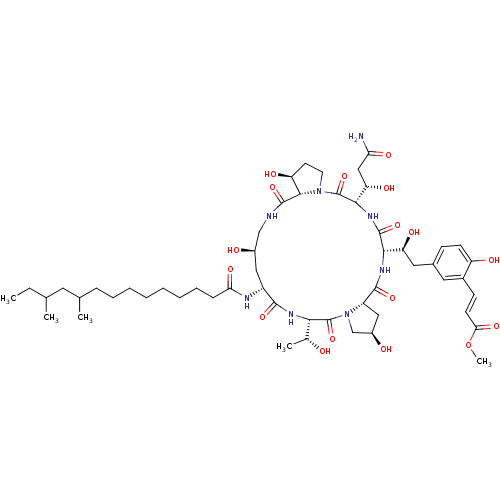
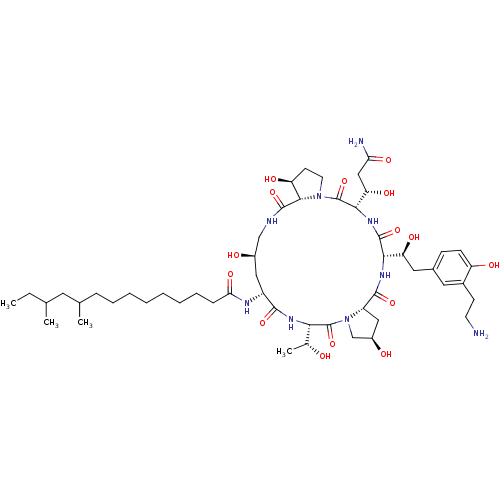
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataKi: 4.80nMAssay Description:Binding affinity to Kv11.1 channel (unknown origin)More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: >500nMAssay Description:Inhibition of ERG in human VCaP cells after 48 hrs by Western blotting analysisMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase component GSC2(Saccharomyces cerevisiae)
Leiden University
Curated by ChEMBL
Leiden University
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:In vitro inhibition of 1,3-beta-glucan synthase in Candida albicans membrane assay.More data for this Ligand-Target Pair