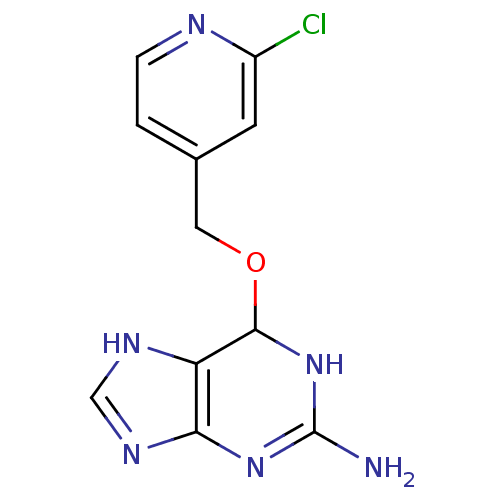
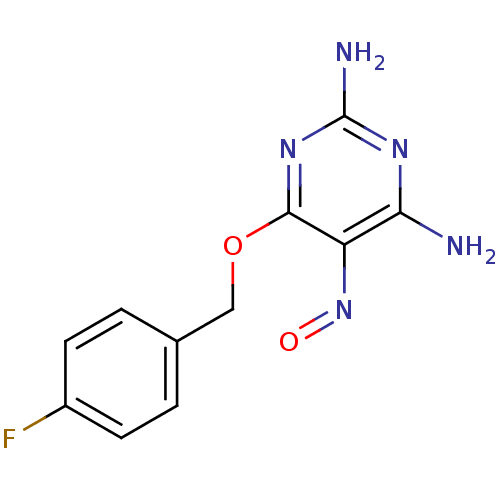
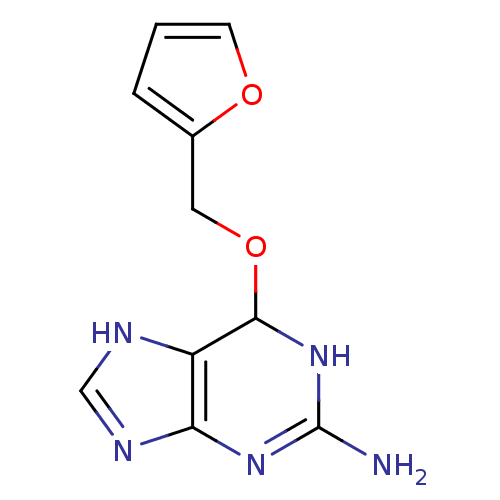
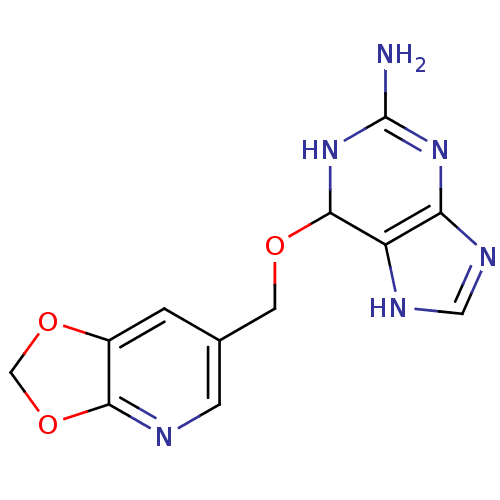
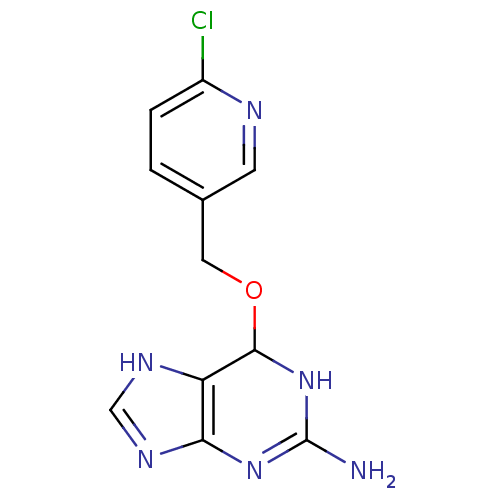
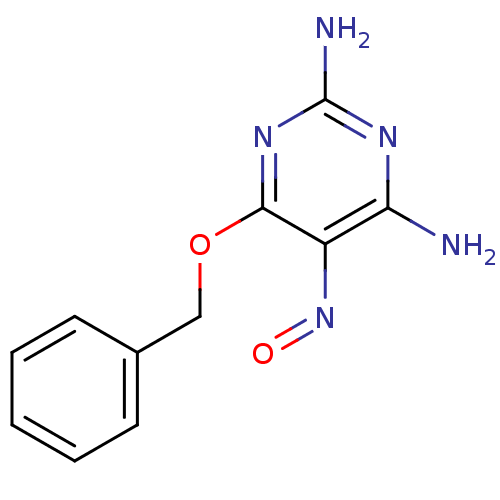
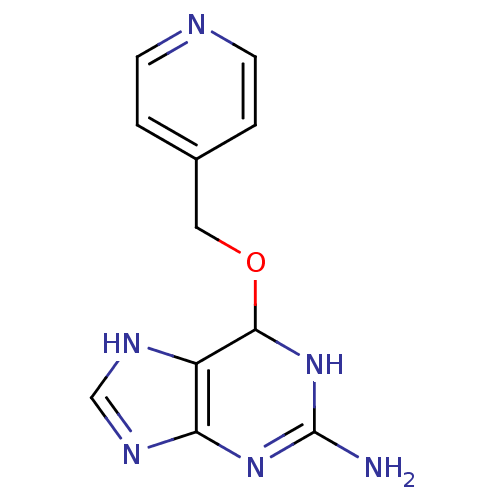
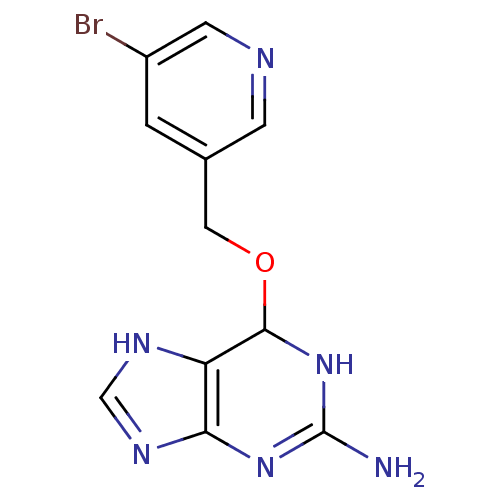
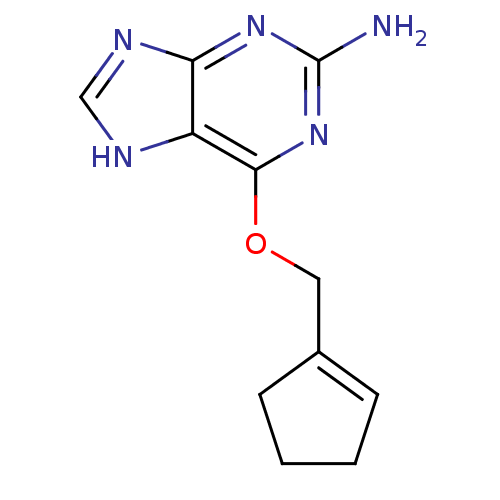
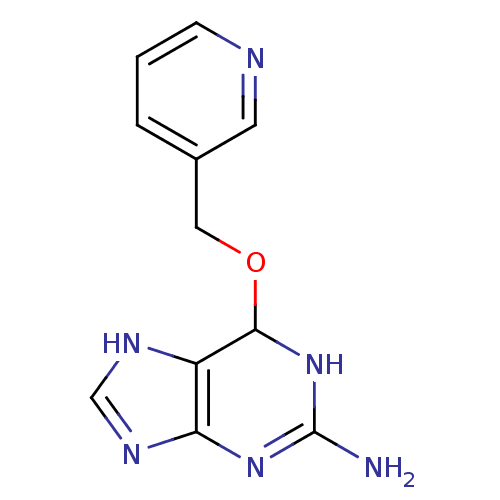
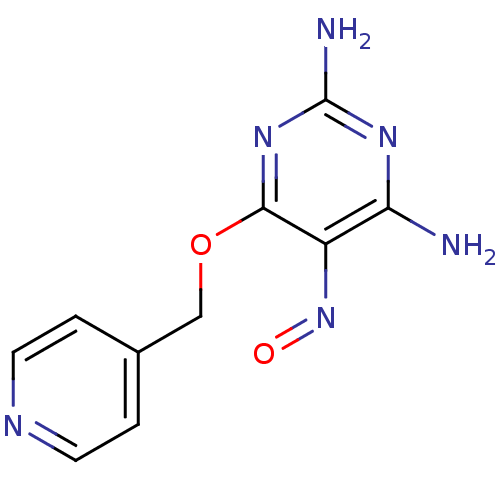
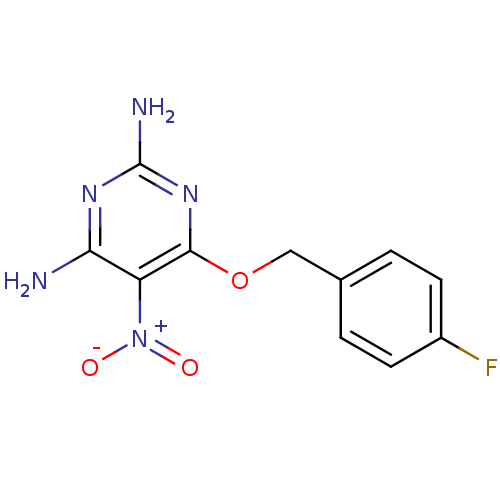
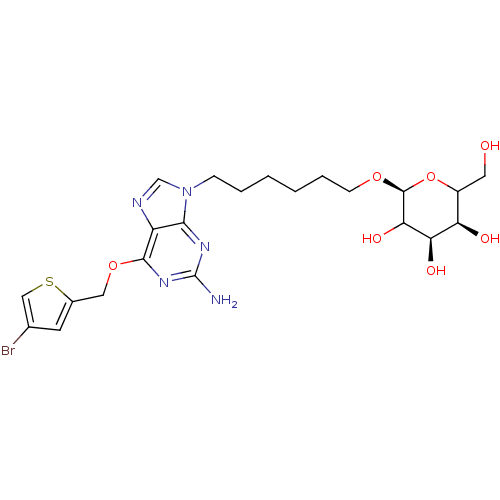
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Inhibition of MGMT (unknown origin)More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of AGT activity to 50% of control rate in HT-29 cell extractMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inactivation of human O-6-methylguanine-DNA methyltransferase assessed as [3H]methylated protein formation by liquid scintillation counting in presen...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:In vitro inhibitory activity against human O6-alkylguanine-DNA alkyltransferase (AGAT)More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 58nMAssay Description:concentration required to reduce AGT activity to 50% of control rate in intact HT-29 human colorectal carcinoma cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of AGT in human HL60 cells assessed as AGT levels using [3H]AGT after 2 hrsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:In vitro inhibitory activity against human O6-alkylguanine-DNA alkyltransferase (AGAT)More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of AGT activity to 50% of control rate in HT-29 cell extractMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:concentration required to reduce AGT activity to 50% of control rate in intact HT-29 human colorectal carcinoma cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity in Raji cells (Concentration of inactivator required to produce 50% reduction in ATPase ac...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:In vitro inhibitory activity against human O6-alkylguanine-DNA alkyltransferase (AGAT)More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inactivation of human O-6-methylguanine-DNA methyltransferase assessed as [3H]methylated protein formation by liquid scintillation counting in presen...More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:In vitro inhibitory activity against human O6-alkylguanine-DNA alkyltransferase (AGAT)More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibition of AGT activity to 50% of control rate in HT-29 cell extractMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:O6-Alkylguanine-DNA Alkyltransferase-Inactivating Activity (Concentration of inactivator required to produce 50% reduction in ATPase activity) More data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
Eberhard Karls University T£Bingen
Curated by ChEMBL
Eberhard Karls University T£Bingen
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair











 3D Structure (crystal)
3D Structure (crystal)