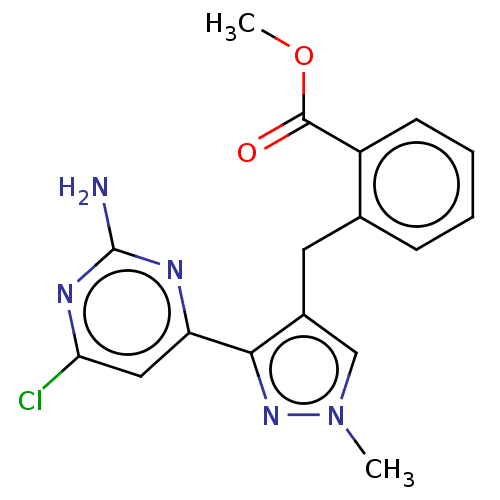
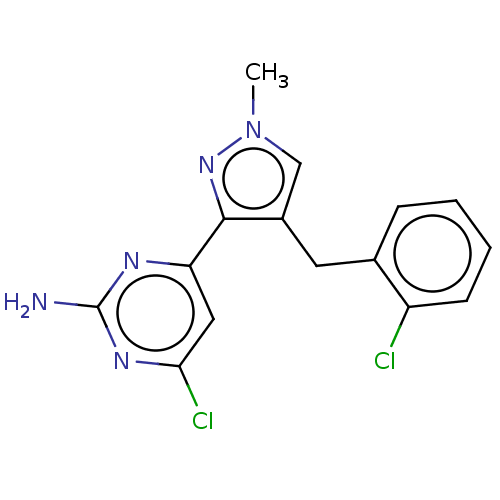
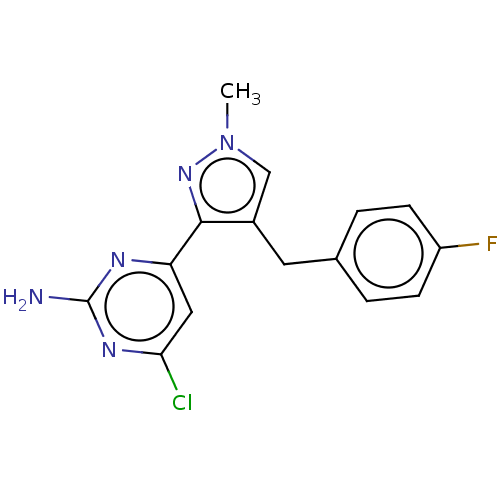
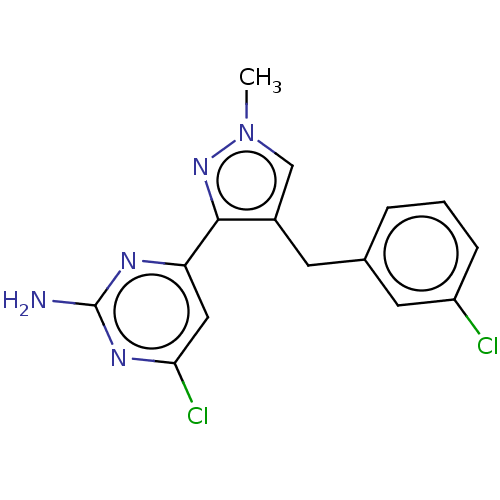
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
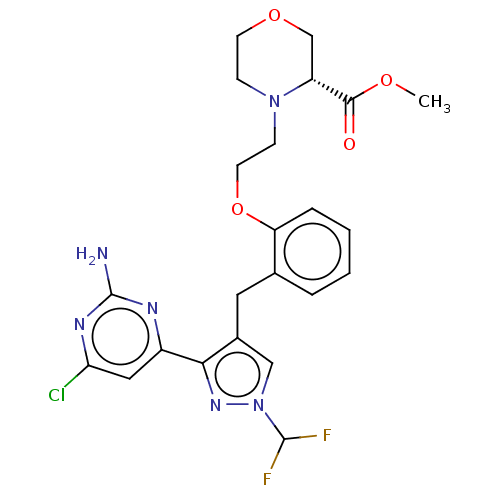
Affinity DataIC50: 1nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
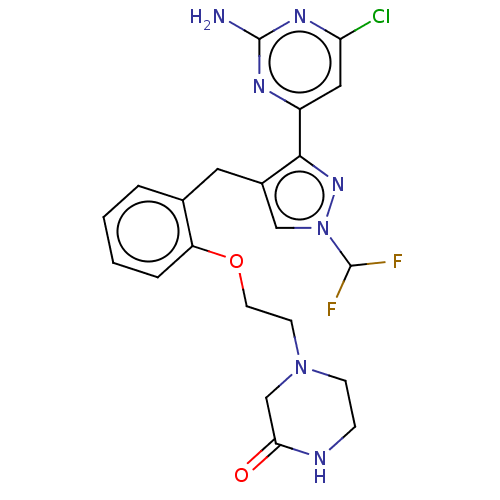
Affinity DataIC50: 1.90nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 6.70nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 7.80nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 64nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 74nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 87nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 92nMAssay Description:Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 102nMAssay Description:Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 107nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 112nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 126nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 132nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 141nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 159nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of human ADCY10More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 195nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 196nMAssay Description:Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 225nMAssay Description:Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 325nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 342nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 351nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 351nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 389nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 392nMAssay Description:Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assayMore data for this Ligand-Target Pair





 3D Structure (crystal)
3D Structure (crystal)