TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 69nMAssay Description:In vitro inhibitory activity against glucagon induced monkey adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 118nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 144nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 254nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 317nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 610nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:In vitro inhibitory activity against glucagon induced human adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:KB for inhibition of adenylate cyclase stimulation in human platelet membranesMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 4.40E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of dopamine-sensitive rat brain adenylyl cyclase activity assessed as cAMP levelMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of dopamine-sensitive rat brain adenylyl cyclase activity assessed as cAMP levelMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 6.70nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 7.80nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Rattus norvegicus)
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of rat ADCY10 overexpressed in rat 4-4 cells assessed as IBMX stimulated cAMP accumulation preincubated for 10 mins followed by IBMX stimu...More data for this Ligand-Target Pair
TargetAdenylate cyclase type 10(Homo sapiens (Human))
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Tri-Institutional Institutional Therapeutics Discovery Institute
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of human ADCY10 assessed as cAMP accumulation preincubated for 15 mins followed by substrate addition using alpha-32P labelled ATP as subs...More data for this Ligand-Target Pair





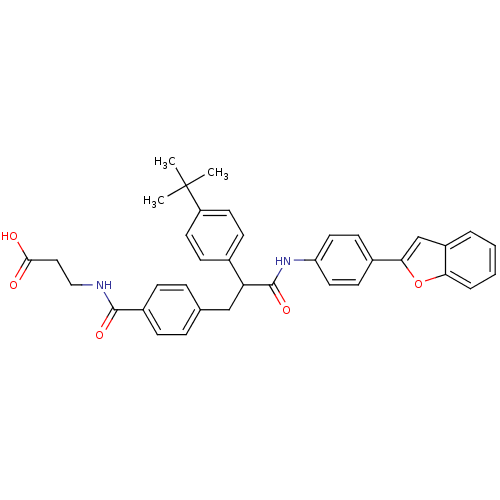

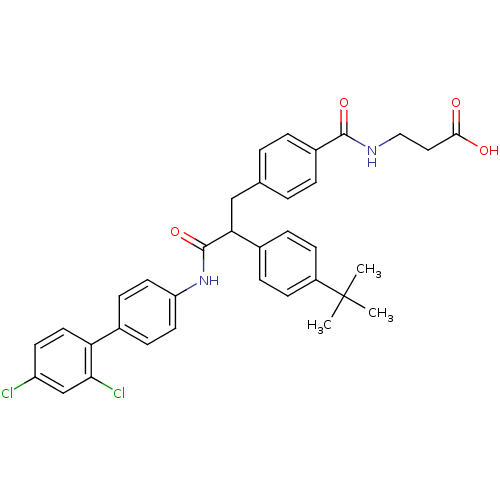

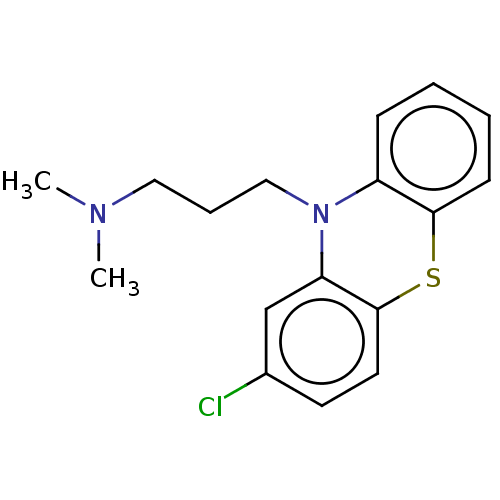
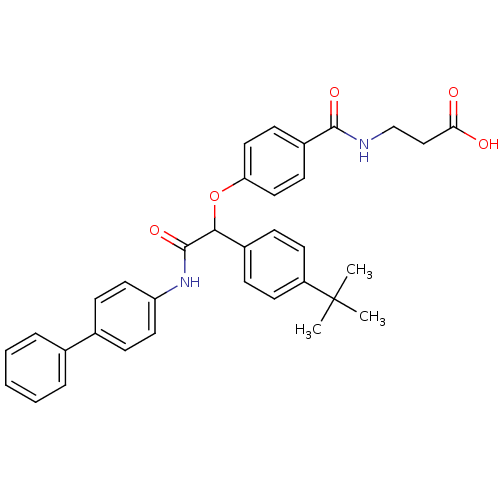




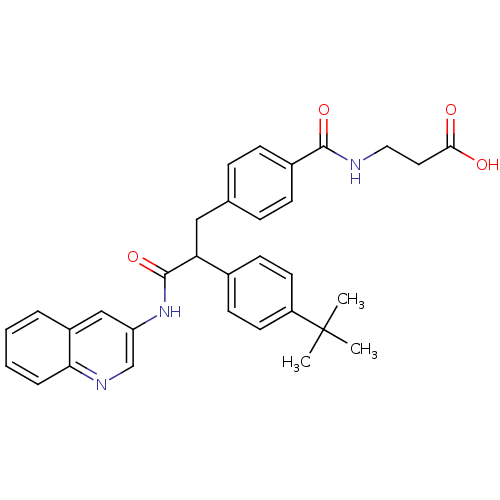

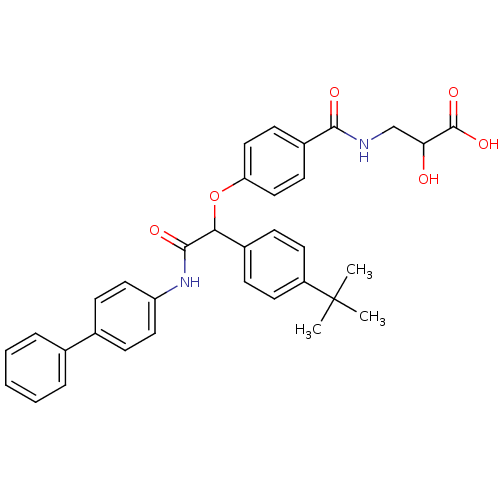

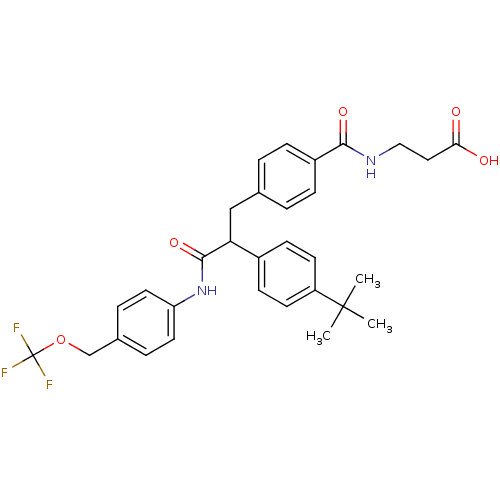
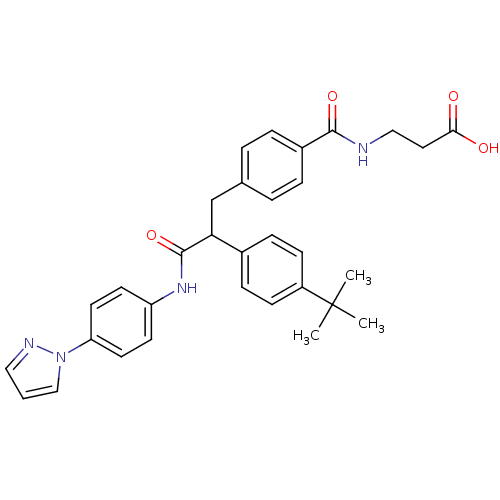





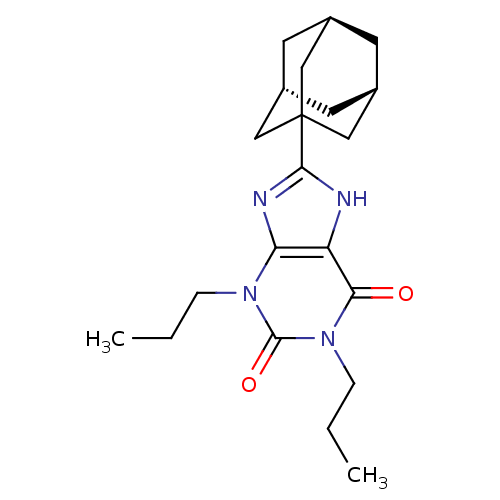

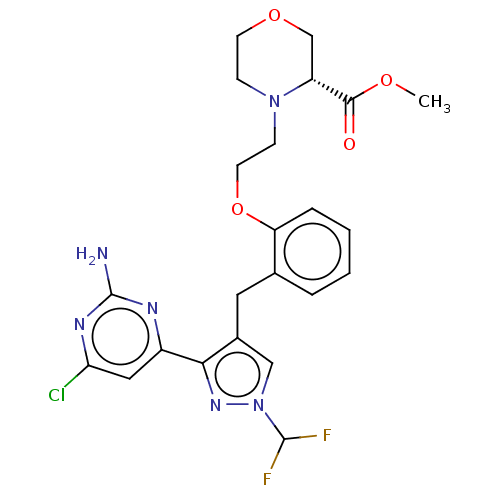
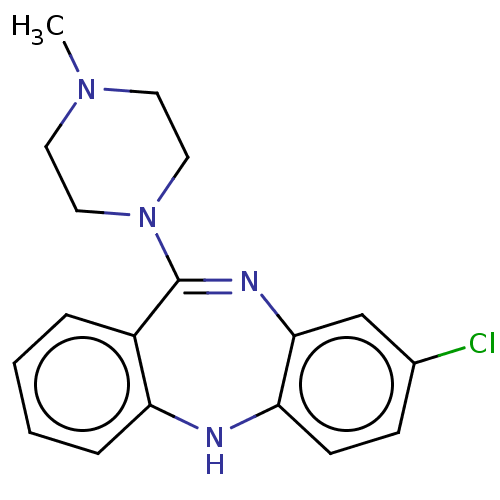



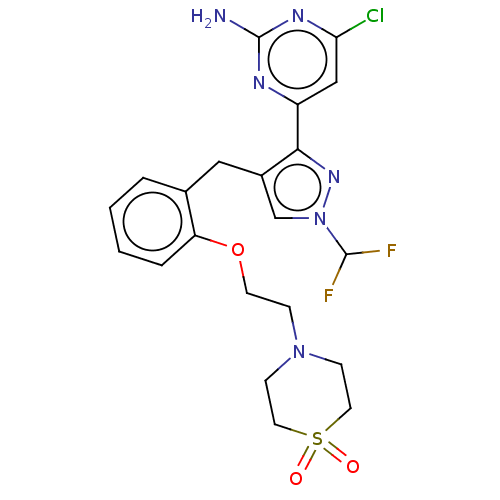

 3D Structure (crystal)
3D Structure (crystal)







