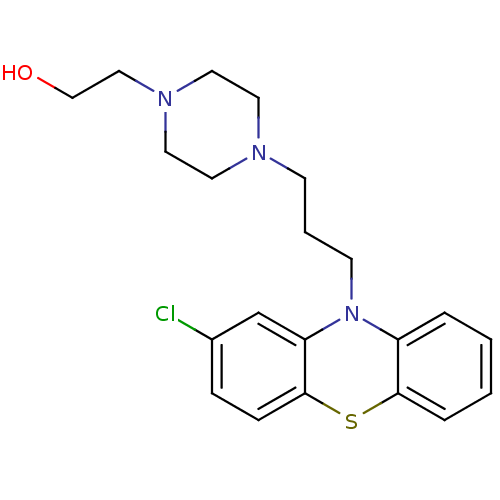
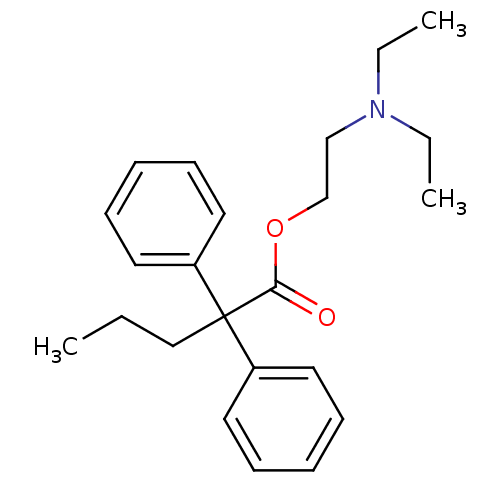
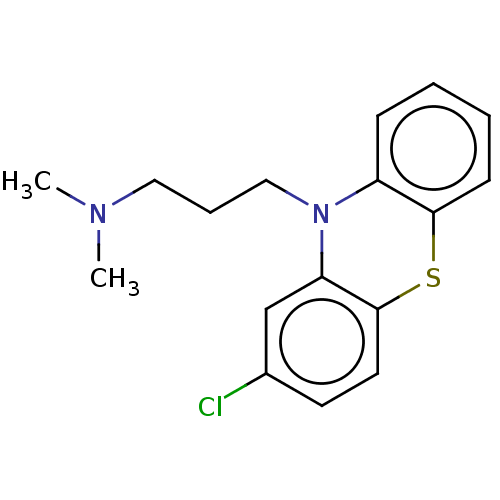
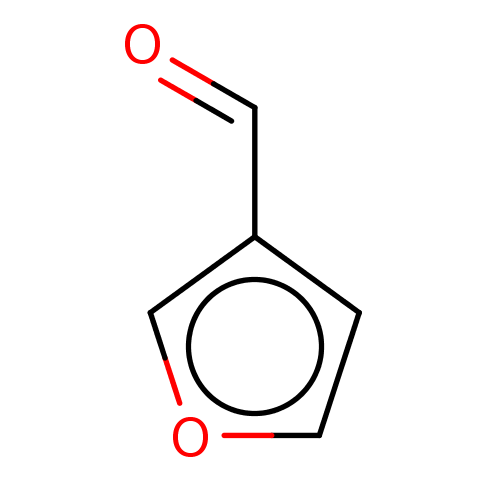
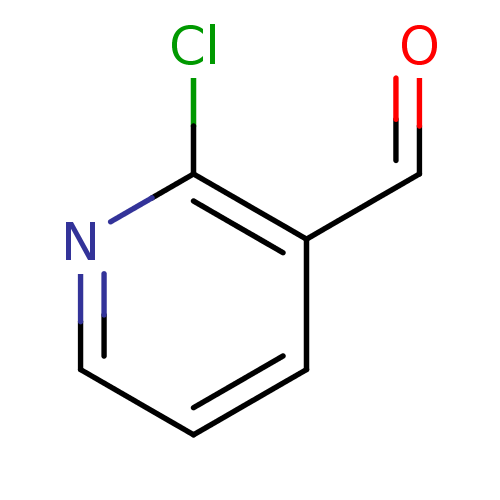
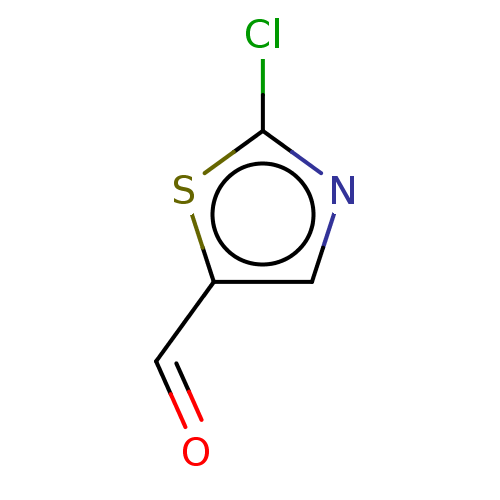
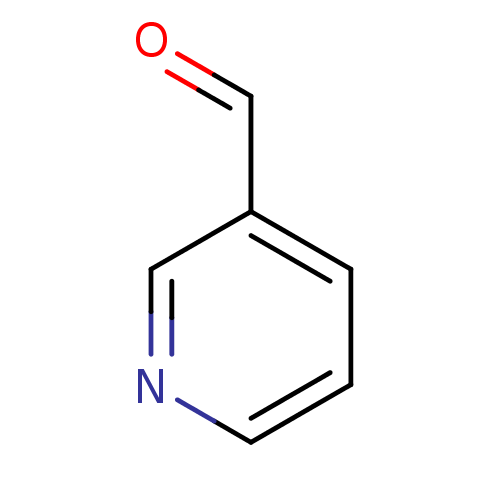
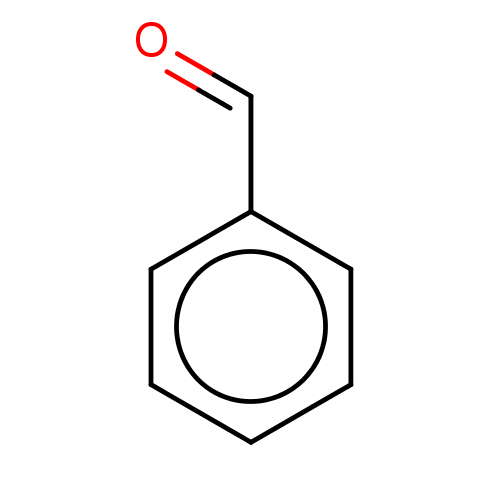
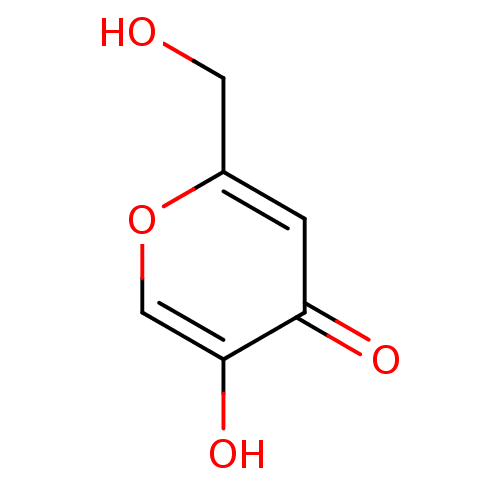
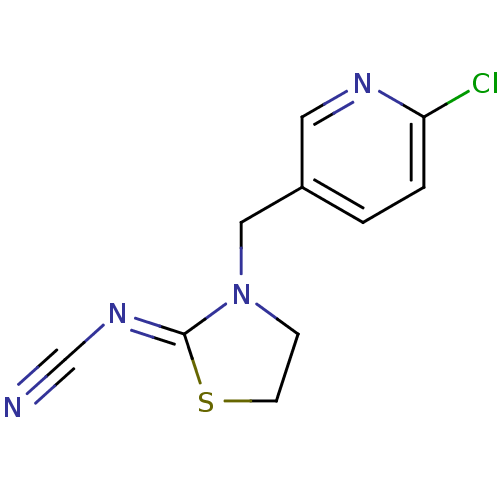
Affinity DataKi: 30nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of rabbit aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition of rabbit aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.53E+3nM IC50: 1.45E+4nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 7.23E+3nM IC50: 3.18E+4nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 4.80E+4nM IC50: 4.94E+4nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 7.21E+4nM IC50: 6.57E+4nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 1.34E+5nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nM IC50: 2.01E+5nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 1.48E+5nM IC50: 2.12E+5nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+5nM IC50: 1.15E+5nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+5nM IC50: 1.71E+5nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 2.53E+5nM IC50: 1.93E+5nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataKi: 4.42E+5nMpH: 7.0Assay Description:Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of monkey aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of mouse aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of monkey aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of mouse aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 740nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of rabbit aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of monkey aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of mouse aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of rabbit aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of monkey aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of rabbit aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of rabbit aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+4nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 7.57E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+5nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of Oryctolagus cuniculus (rabbit) AOX in liver cytosolMore data for this Ligand-Target Pair



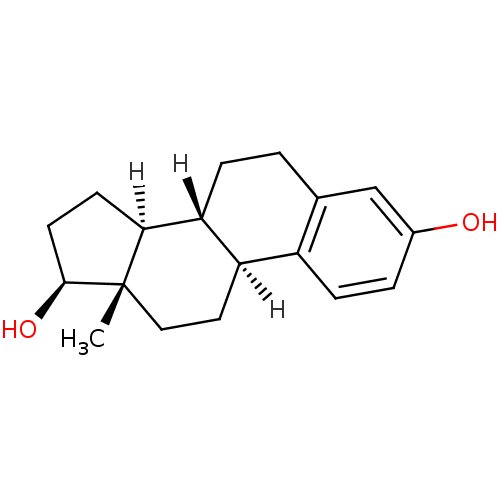

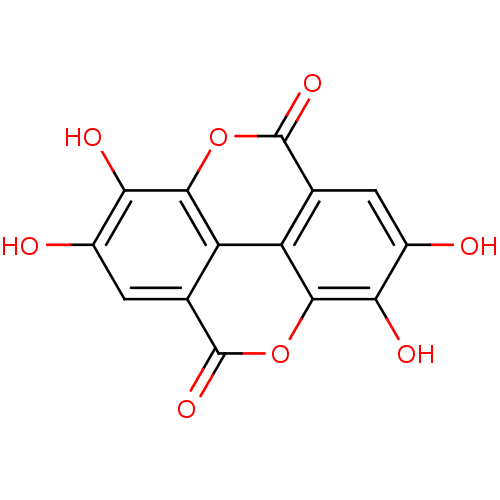









 3D Structure (crystal)
3D Structure (crystal)