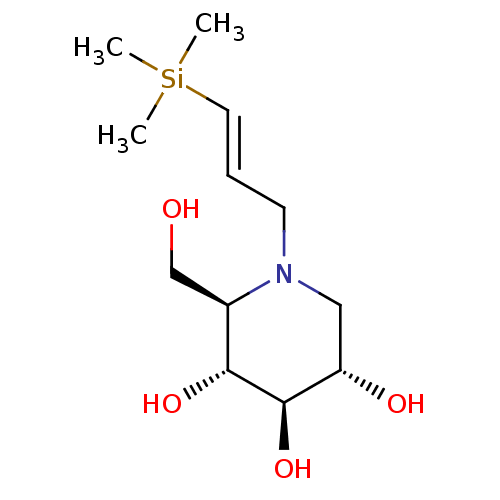
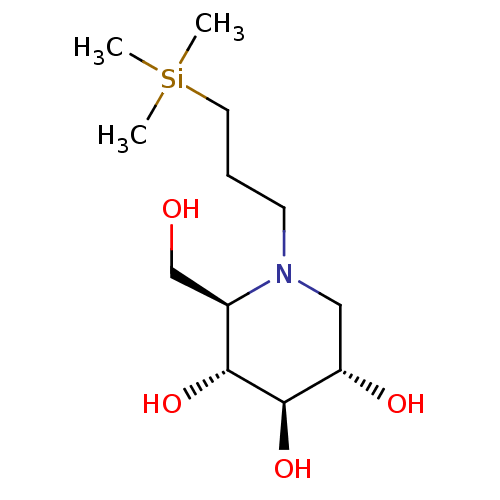
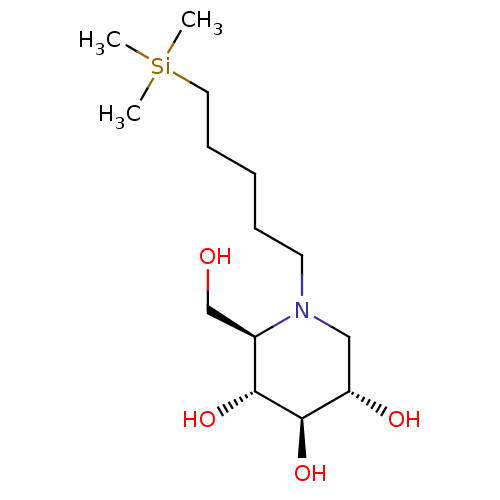
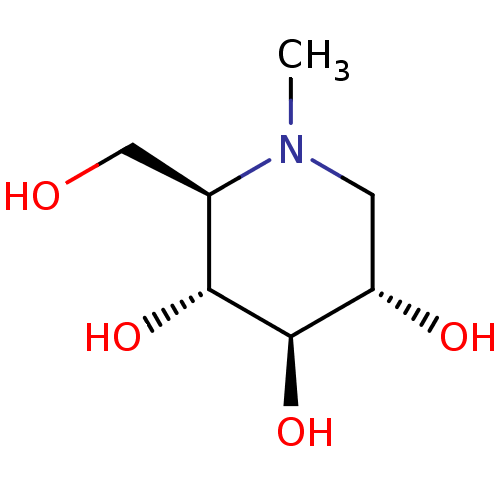
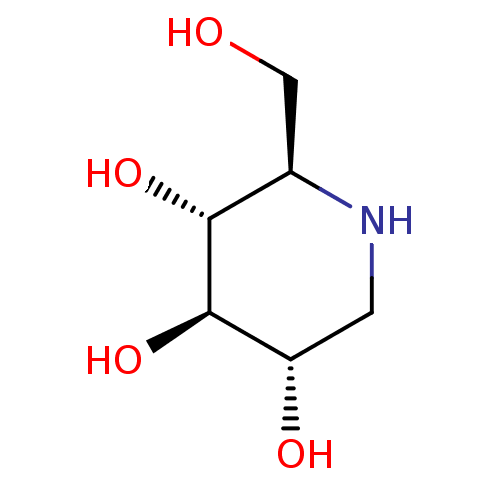
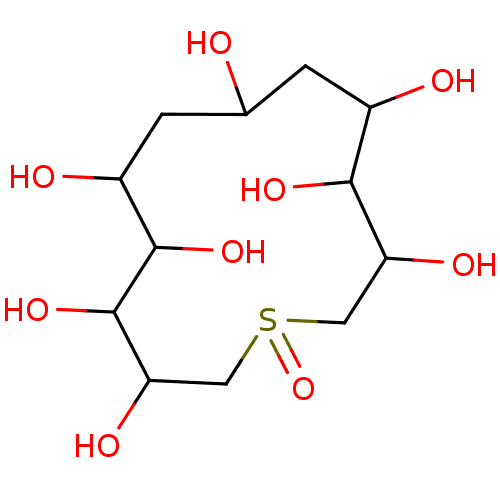
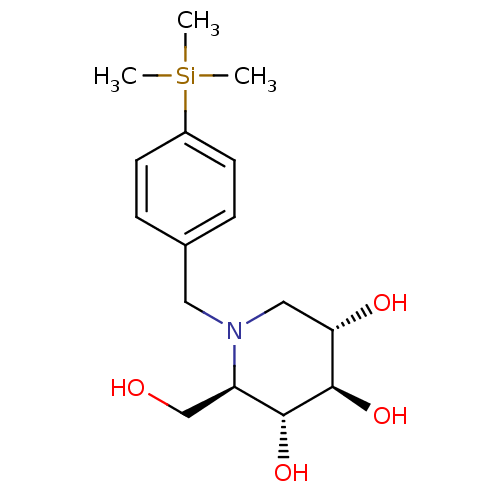
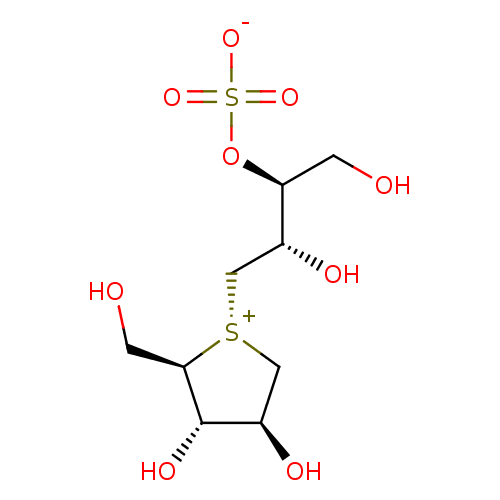
Affinity DataKi: 0.150nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 64nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 103nMAssay Description:Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 185nMAssay Description:Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 216nMAssay Description:Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 302nMAssay Description:Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 353nMAssay Description:Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 390nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 640nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 785nMAssay Description:Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 830nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.30E+3nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 3.50E+3nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 4.20E+3nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.20E+3nMAssay Description:Inhibition of sucrose hydrolyzing activity of rat intestinal alpha glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.20E+3nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataKi: 8.50E+3nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 1.15E+4nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal isomaltaseMore data for this Ligand-Target Pair