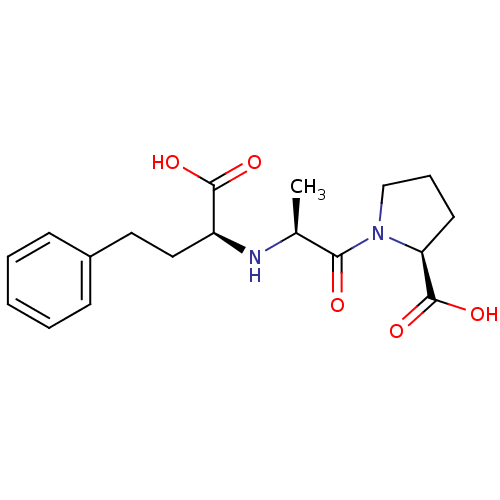
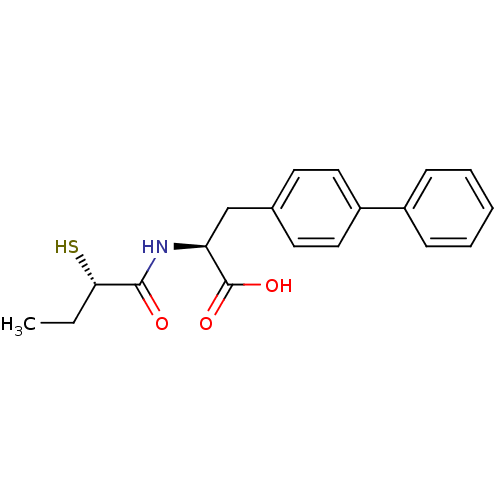
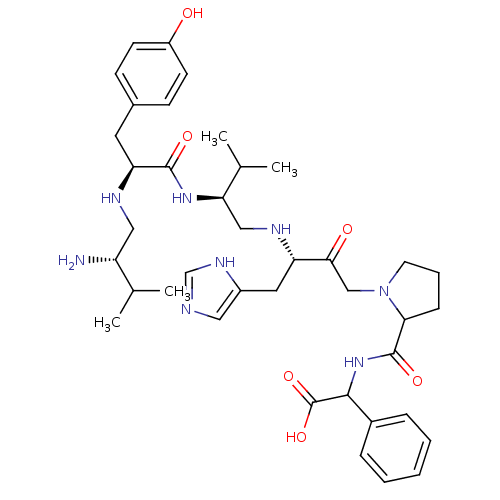
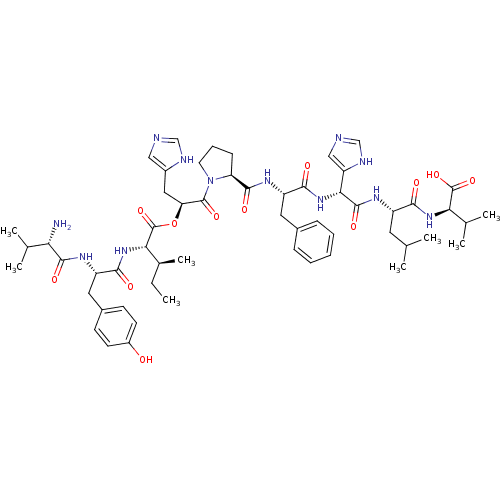
Affinity DataKi: 0.130nM ΔG°: -56.4kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nM ΔG°: -53.6kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity towards Angiotensin I converting enzyme of rat brain IgG immobilized enzyme.More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -52.3kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 1.25nM ΔG°: -50.8kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Binding affinity to angiotensin converting enzyme 2More data for this Ligand-Target Pair
Affinity DataKi: 5.20nM ΔG°: -47.3kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 5.20nM ΔG°: -47.3kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 6.5nM ΔG°: -46.7kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 6.60nM ΔG°: -46.7kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 6.90nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 7nM ΔG°: -46.5kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 7.10nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nM ΔG°: -46.4kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 84nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 220nM ΔG°: -38.0kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 300nM ΔG°: -37.2kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 300nM ΔG°: -37.2kJ/molepH: 6.8 T: 2°CAssay Description:Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra...More data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 550nMAssay Description:Inhibition of human recombinant ACE2 by fluorescence assayMore data for this Ligand-Target Pair