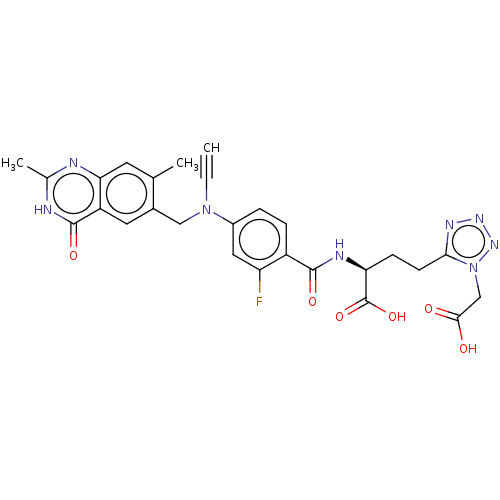
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Competitive inhibition of human 15-LOX-2 assessed as equilibrium dissociation constant from catalytic site by measuring 15-HpETE by Dixon plots and D...More data for this Ligand-Target Pair
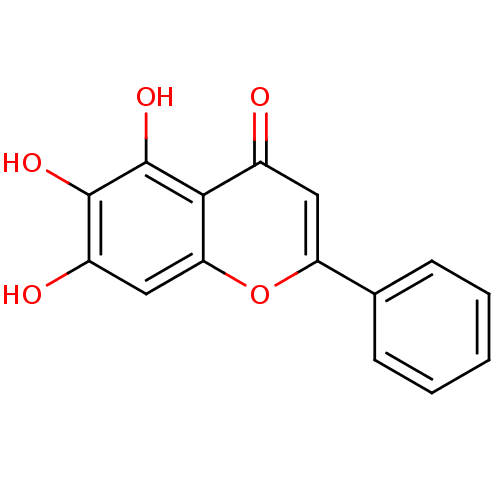
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataKi: 800nMAssay Description:Competitive inhibition of human 15-LOX-2 assessed as equilibrium dissociation constant from catalytic site by measuring 15-HpETE by Dixon plots and D...More data for this Ligand-Target Pair
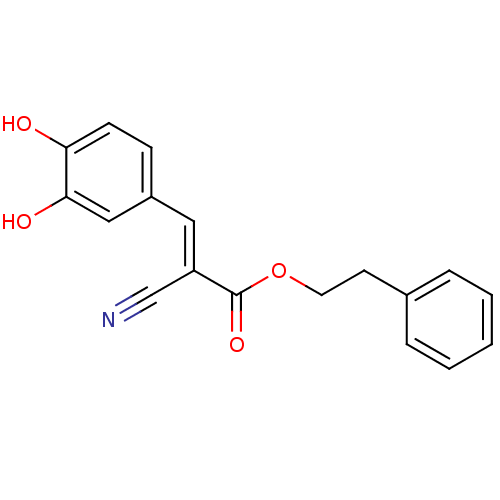
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Competitive inhibition of human 15-LOX-2 assessed as equilibrium dissociation constant from catalytic site by measuring 15-HpETE by Dixon plots and D...More data for this Ligand-Target Pair
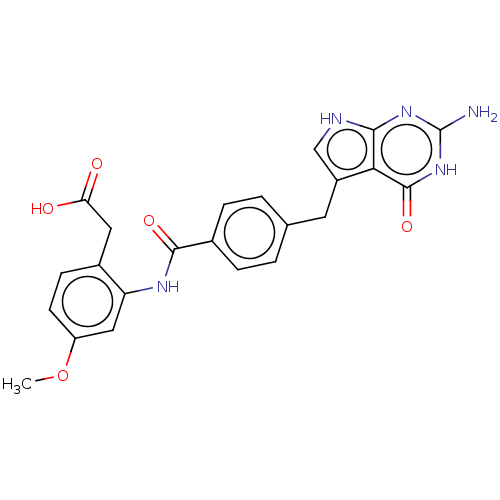
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Competitive inhibition of human 15-LOX-2 assessed as equilibrium dissociation constant from catalytic site by measuring 15-HpETE by Dixon plots and D...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataKi: 2.50E+3nMAssay Description:Competitive inhibition of human 15-LOX-2 assessed as equilibrium dissociation constant from catalytic site by measuring 15-HpETE by Dixon plots and D...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of thymidylate synthase (unknown origin)More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibition of thymidylate synthase (unknown origin)More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytesMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytesMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of human 15-LOX-2 assessed as enzymatic rate using arachidonic acid as substrate by UV/Vis spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytesMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 480nMAssay Description:Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytesMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataIC50: 530nMAssay Description:Inhibition of human 15-LOX-2 assessed as enzymatic rate using arachidonic acid as substrate by UV/Vis spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 560nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 740nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Homo sapiens (Human))
University Of California Santa Cruz
Curated by ChEMBL
University Of California Santa Cruz
Curated by ChEMBL
Affinity DataIC50: 870nMAssay Description:Inhibition of human 15-LOX-2 assessed as enzymatic rate using arachidonic acid as substrate by UV/Vis spectrophotometric analysisMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 870nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.28E+3nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytesMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.58E+3nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.62E+3nMAssay Description:Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytesMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.65E+3nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 1.97E+3nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 2.04E+3nMAssay Description:Inhibition of human thymidylate synthase using dUMP as substrate by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of recombinant human His6-tagged TS using dUMP and 5,10-methylenetetrahydrofolate as substrate by spectrometryMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 2.21E+3nMAssay Description:Inhibition of human thymidylate synthase expressed in Escherichia coli using 6R,S-tetrahydrofolate and dUMP as substrate by spectrophotometric analys...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Rattus norvegicus)
Henan University Of Chinese Medicine
Curated by ChEMBL
Henan University Of Chinese Medicine
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric methodMore data for this Ligand-Target Pair






 3D Structure (crystal)
3D Structure (crystal)