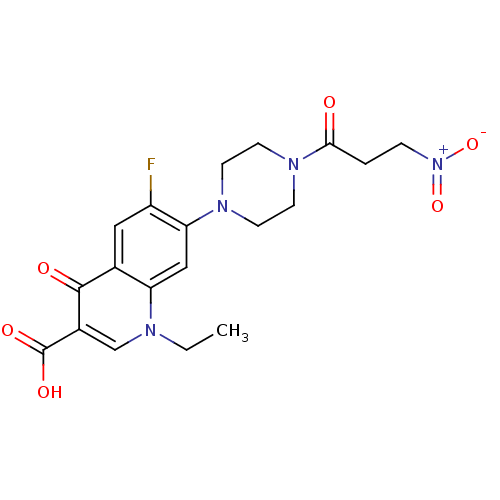
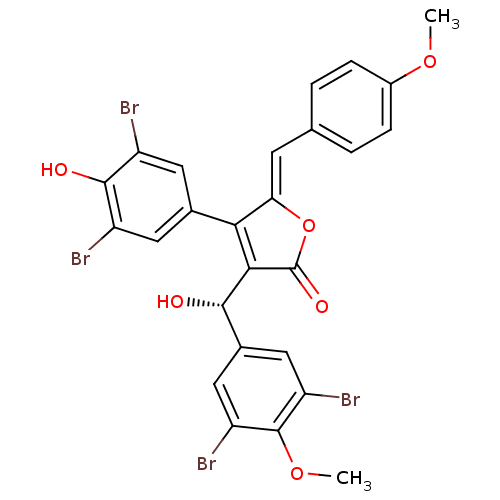
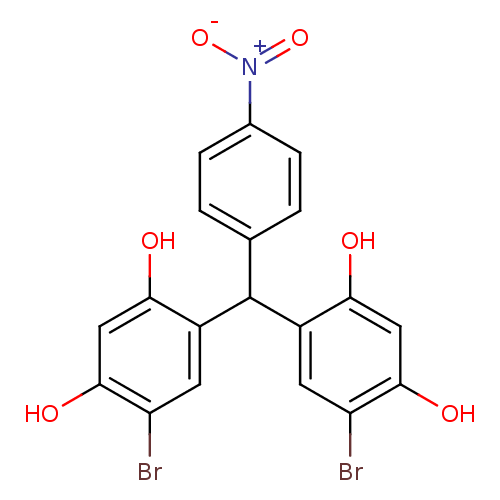
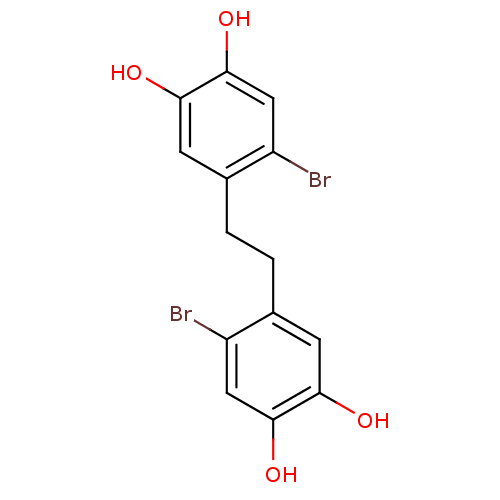
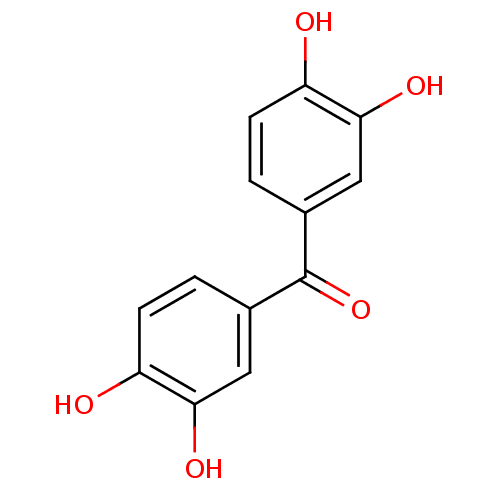
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
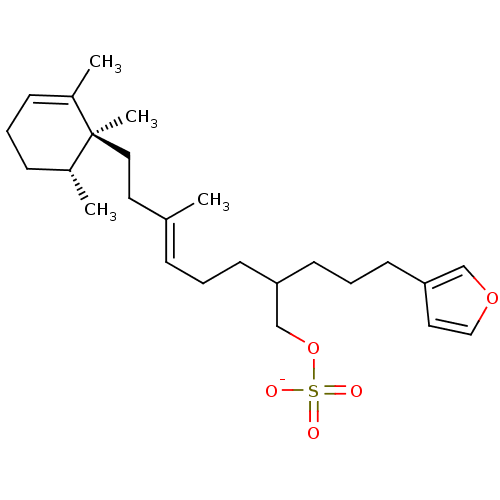
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
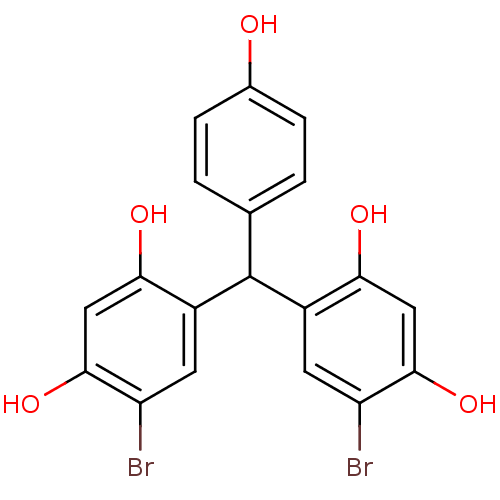
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
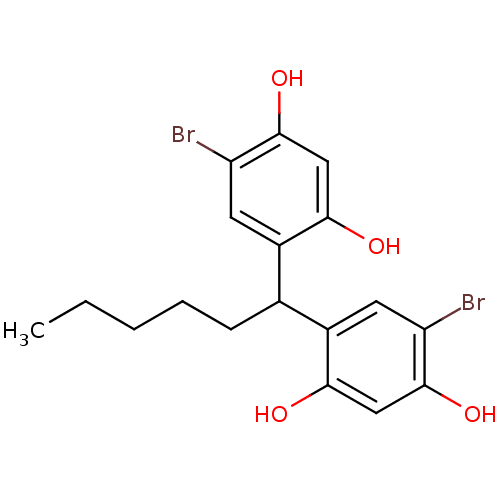
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 1.46E+3nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.65E+3nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of Candida albicans isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of Candida albicans isocitrate lyase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.62E+3nMAssay Description:Inhibition of recombinant Candida albicans ATCC 10231 ICL using phenylhydrazine and isocitrate as substrate assessed as formation of glyoxylate pheny...More data for this Ligand-Target Pair
Affinity DataIC50: 8.89E+3nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+3nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyase by spectrophotmeric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of Candida albicans isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of recombinant Candida albicans ATCC 10231 ICL using phenylhydrazine and isocitrate as substrate assessed as formation of glyoxylate pheny...More data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyase by spectrophotmeric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of Candida albicans ICL assessed as reduction in glyoxylate phenylhydrazone formation using M threo-DL(+)isocitrate as substrate incubated...More data for this Ligand-Target Pair
Affinity DataIC50: 1.16E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Magnaporthe oryzae (strain 70-15 / FGSC 8958) (Ric...)
Korea Ocean Research And Development Institute
Curated by ChEMBL
Korea Ocean Research And Development Institute
Curated by ChEMBL
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of Magnaporthe grisea Guy 11 isocitrate lyaseMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.39E+4nMAssay Description:Inhibition of recombinant Candida albicans ATCC 10231 ICL using phenylhydrazine and isocitrate as substrate assessed as formation of glyoxylate pheny...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Magnaporthe oryzae (strain 70-15 / FGSC 8958) (Ric...)
Korea Ocean Research And Development Institute
Curated by ChEMBL
Korea Ocean Research And Development Institute
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of Magnaporthe grisea Guy 11 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of Candida albicans ICL assessed as reduction in glyoxylate phenylhydrazone formation using M threo-DL(+)isocitrate as substrate incubated...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of Candida albicans ICL assessed as reduction in glyoxylate phenylhydrazone formation using M threo-DL(+)isocitrate as substrate incubated...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMAssay Description:Inhibition of recombinant Candida albicans ATCC 10231 ICL using phenylhydrazine and isocitrate as substrate assessed as formation of glyoxylate pheny...More data for this Ligand-Target Pair
Affinity DataIC50: 1.73E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.79E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.84E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.84E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.07E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.12E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 2.13E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of Candida albicans ICL assessed as reduction in glyoxylate phenylhydrazone formation using M threo-DL(+)isocitrate as substrate incubated...More data for this Ligand-Target Pair
Affinity DataIC50: 2.26E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.59E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.63E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyase by spectrophotmeric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.68E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibition of Candida albicans ICL assessed as reduction in glyoxylate phenylhydrazone formation using M threo-DL(+)isocitrate as substrate incubated...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.81E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.01E+4nMAssay Description:Inhibition of Candida albicans isocitrate lyaseMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 3.02E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.09E+4nMAssay Description:Inhibition of Candida albicans ATCC 10231 isocitrate lyaseMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 3.09E+4nMAssay Description:Inhibition of recombinant Mycobacterium tuberculosis ICL expressed in Escherichia coli BL21 (DE3) cells using DL-isocitric acid trisodium salt hydrat...More data for this Ligand-Target Pair
Affinity DataIC50: 3.13E+4nMAssay Description:Inhibition of candida albicans isocitrate lyaseMore data for this Ligand-Target Pair
TargetIsocitrate lyase(Mycobacterium tuberculosis)
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Institute Of Technology And Science-Pilani
Curated by ChEMBL
Affinity DataIC50: 3.25E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis isocitrate lyaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.38E+4nMAssay Description:Inhibition of candida albicans isocitrate lyaseMore data for this Ligand-Target Pair