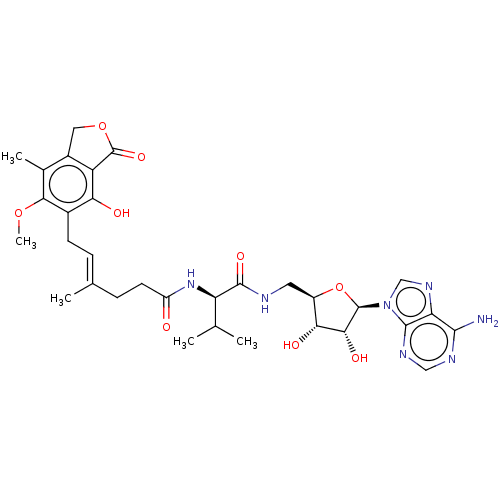
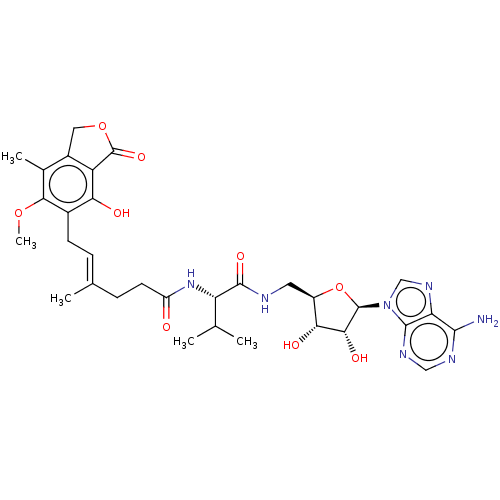
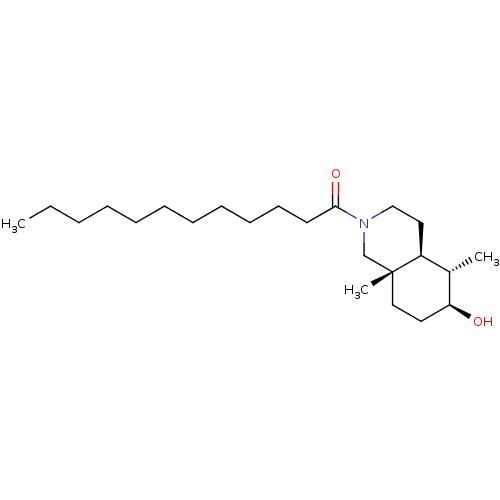
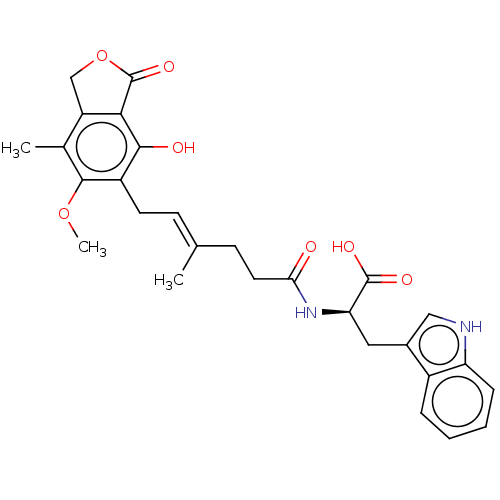
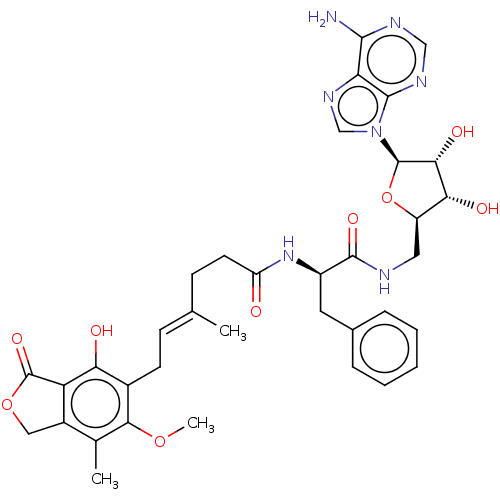
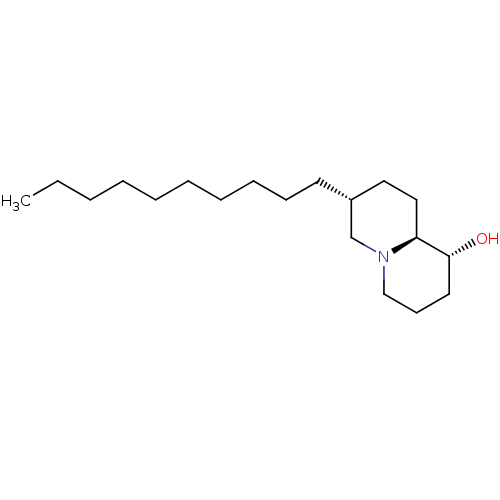
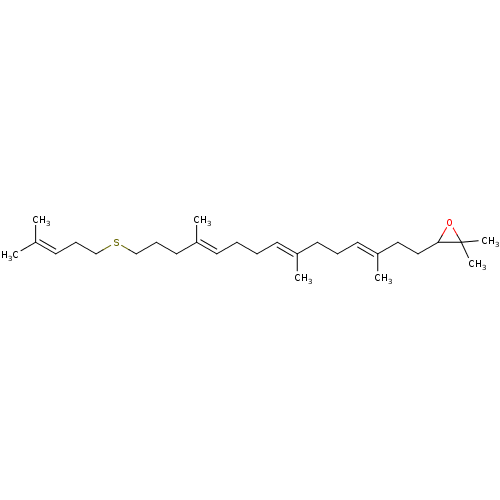
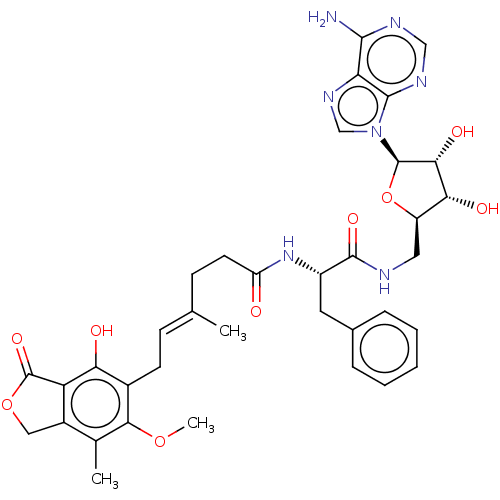
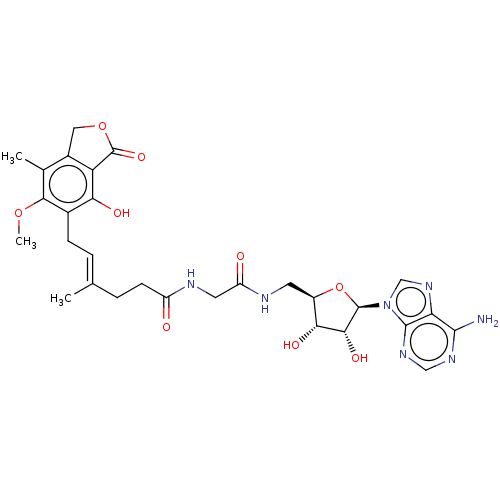
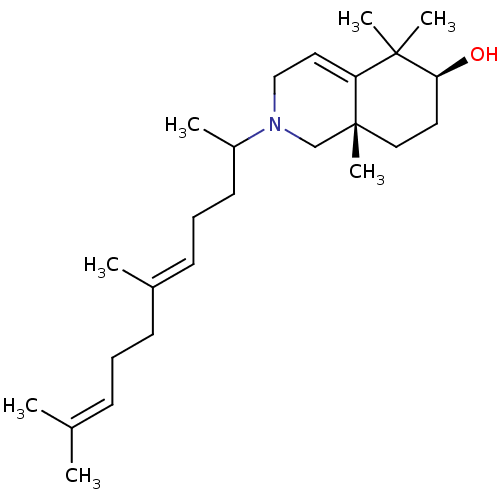
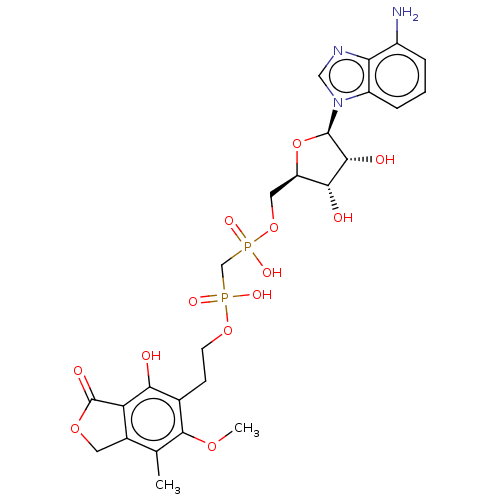
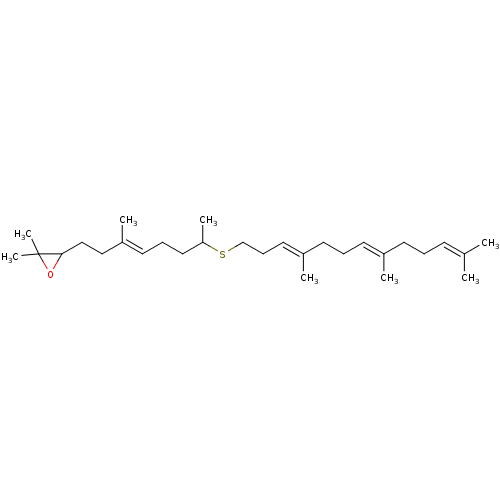
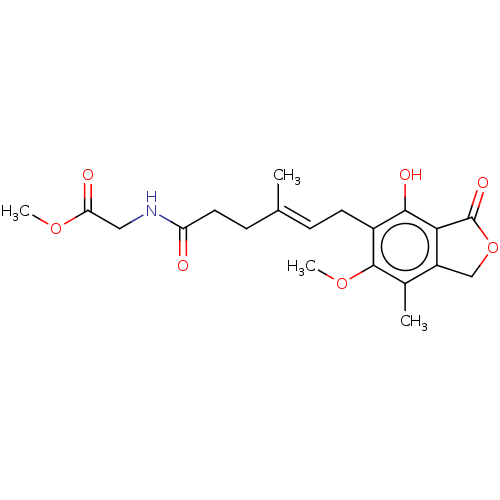
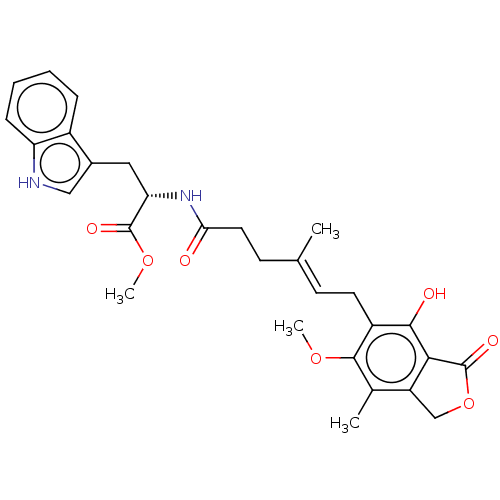
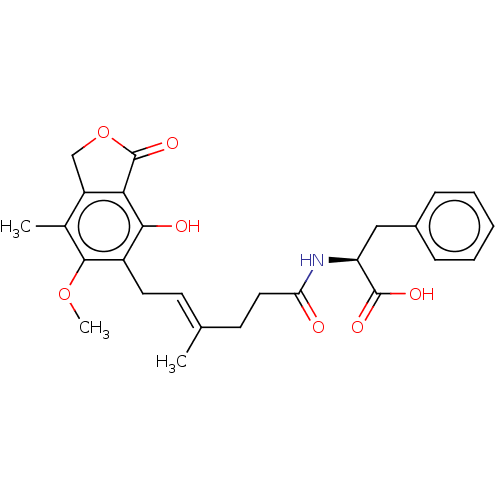
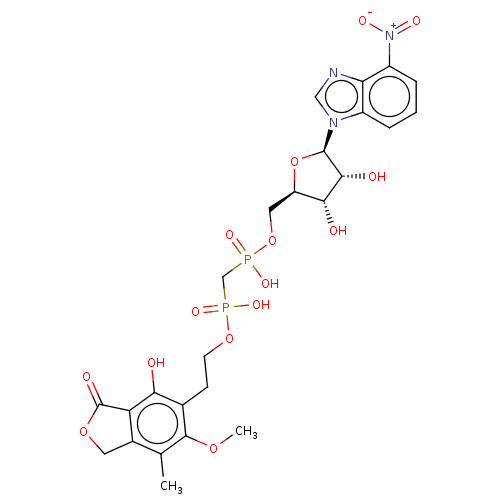
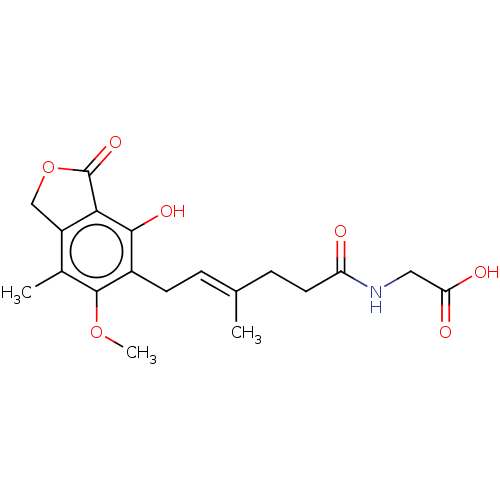
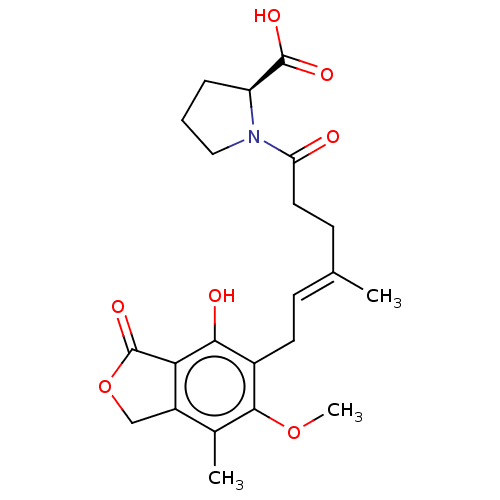
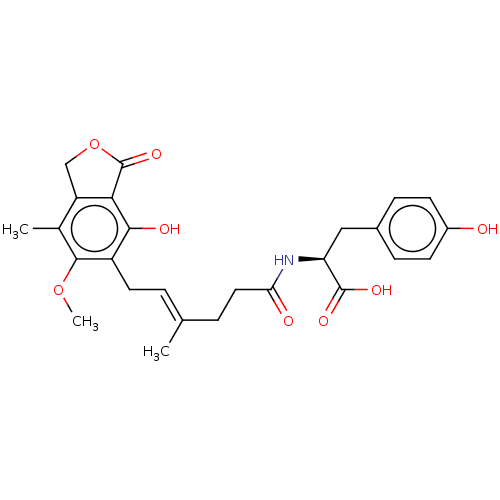
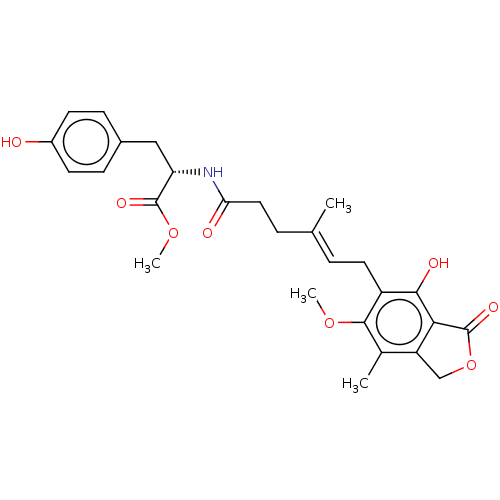
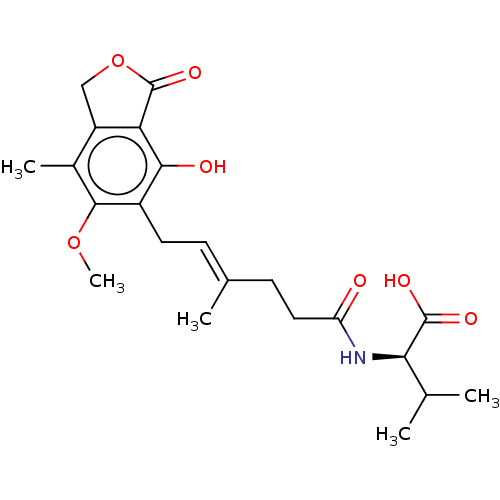
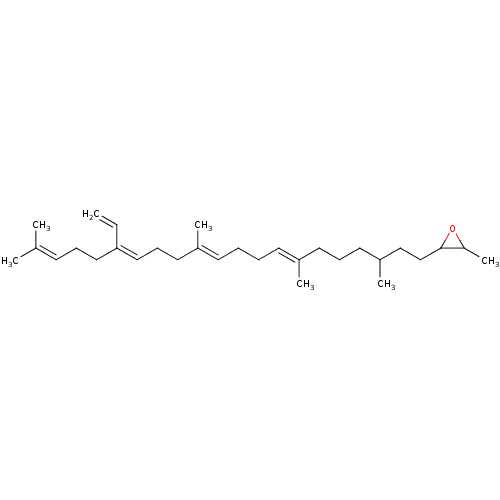
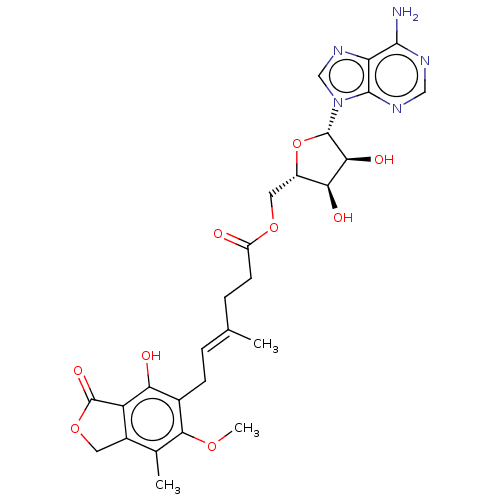
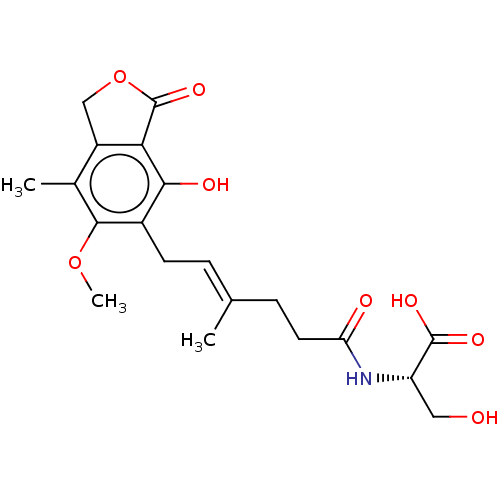
Affinity DataKi: 0.600nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibitory concentration of the compound against purified rat liver Oxidosqualene cyclase (OSC)More data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as KiMore data for this Ligand-Target Pair
Affinity DataKi: 132nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 144nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 163nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 164nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 259nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 259nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 265nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Binding affinity towards competitive inhibition against rat microsomal Oxidosqualene-lanosterol cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity towards noncompetetive inhibition against rat microsomal Oxidosqualene-lanosterol cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 491nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liverMore data for this Ligand-Target Pair
Affinity DataKi: 510nMAssay Description:Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as KiMore data for this Ligand-Target Pair
Affinity DataKi: 522nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 733nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 854nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 876nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 892nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 906nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.02E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liverMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liverMore data for this Ligand-Target Pair
Affinity DataKi: 1.68E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.83E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.04E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Compound was evaluated for the binding affinity against rat liver oxidosqualene cyclase (OSC)More data for this Ligand-Target Pair
Affinity DataKi: 2.55E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.81E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.77E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.31E+3nMAssay Description:Inhibition of IMPDH1 (unknown origin)More data for this Ligand-Target Pair