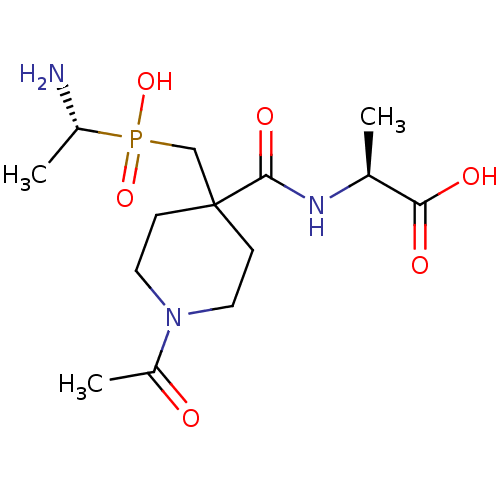
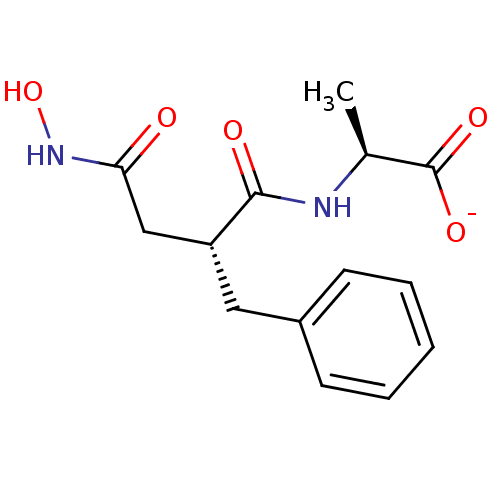
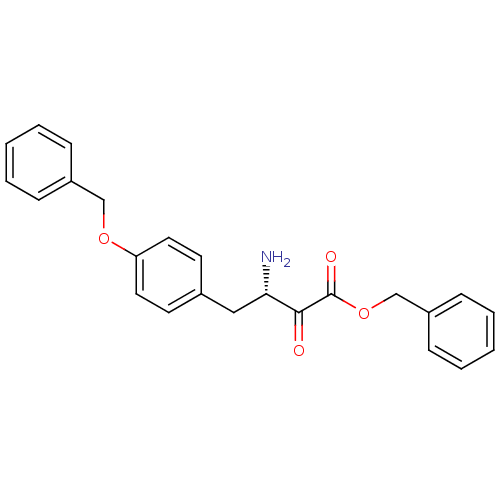
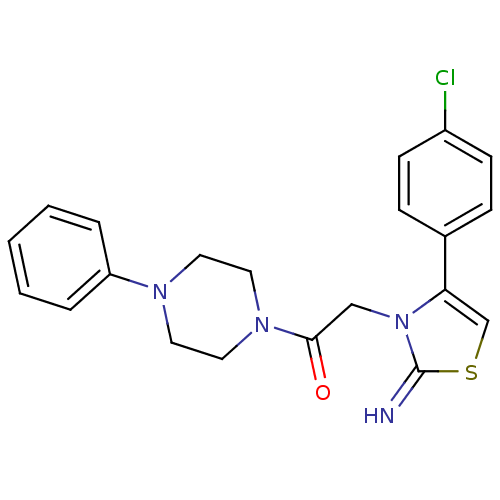
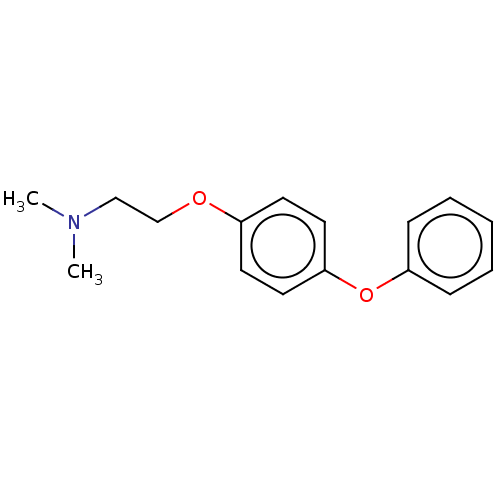
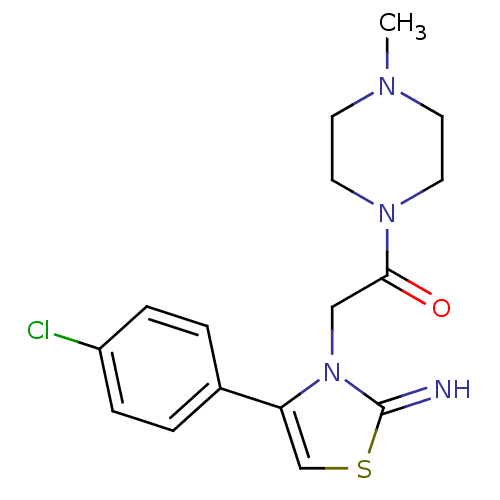
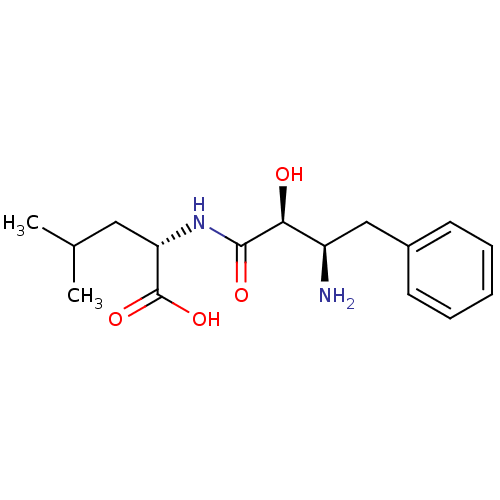
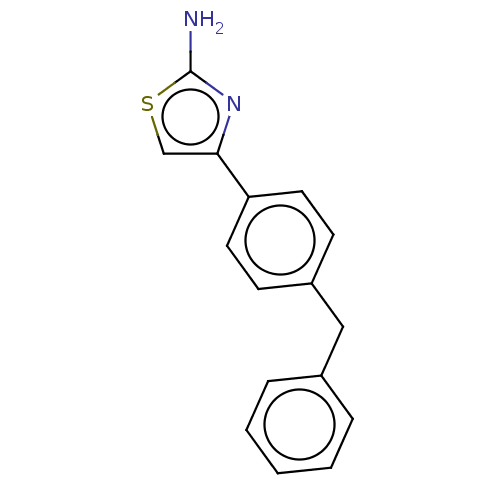
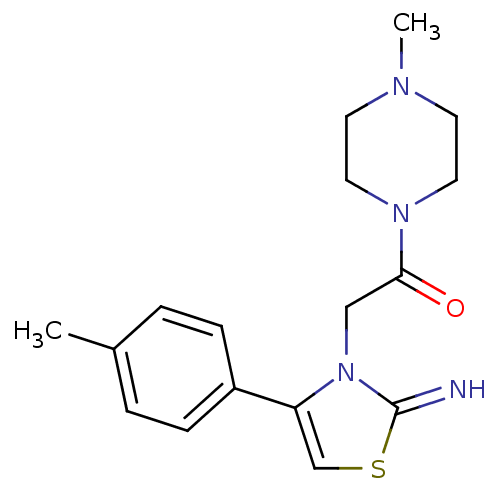
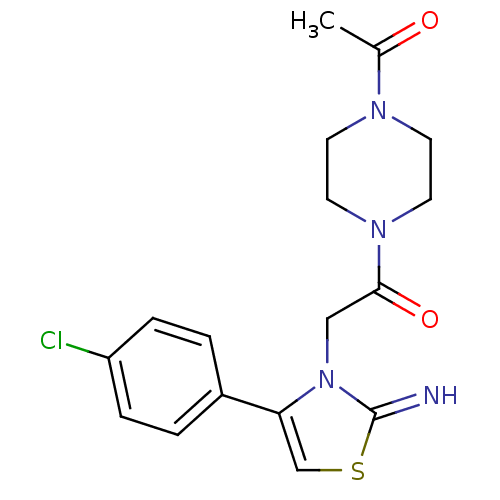
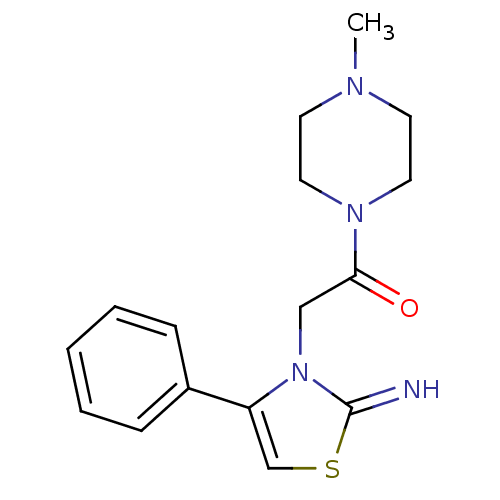
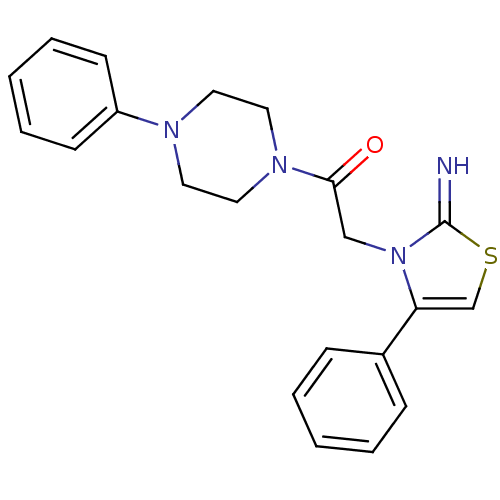
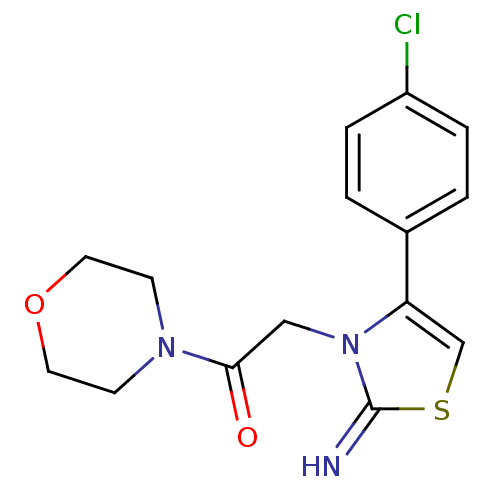
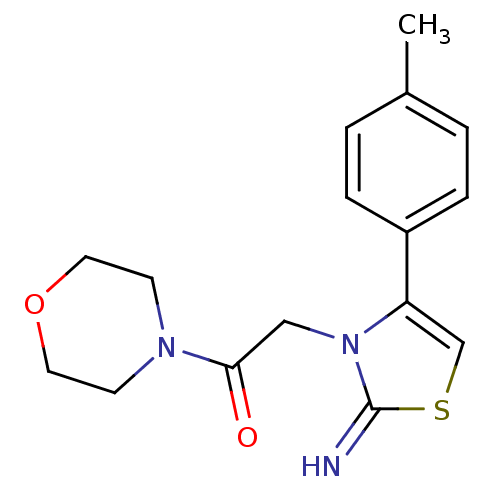
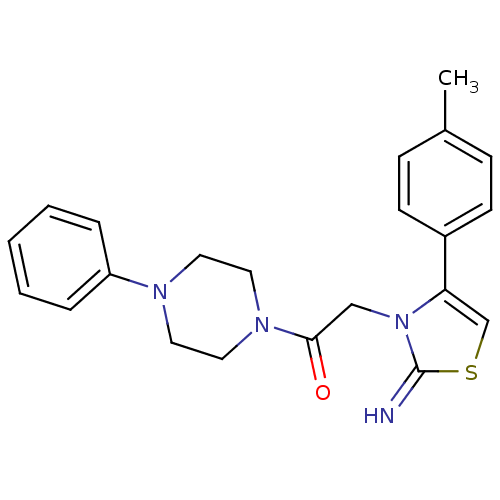
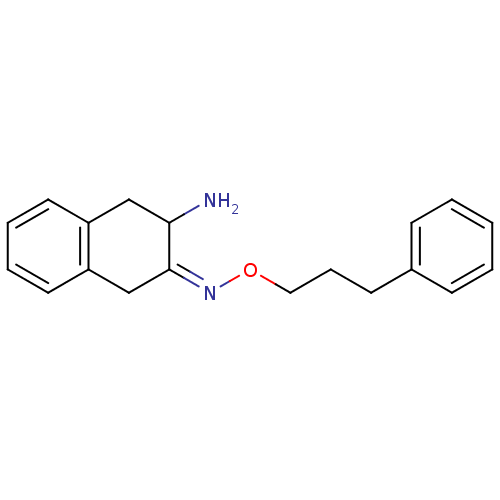
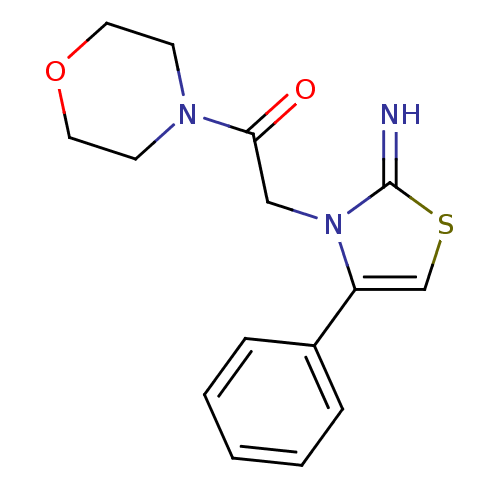
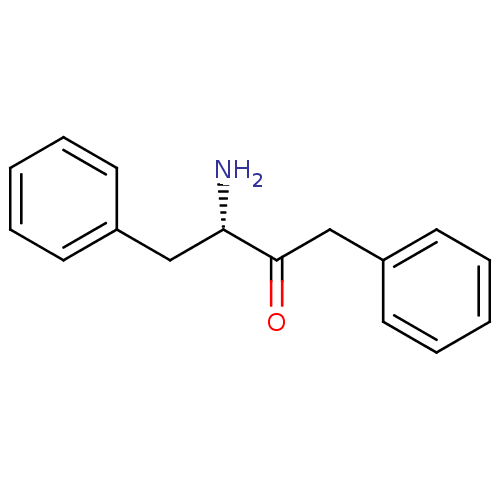
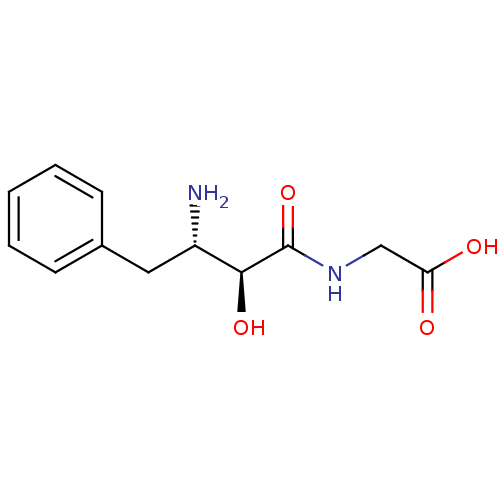
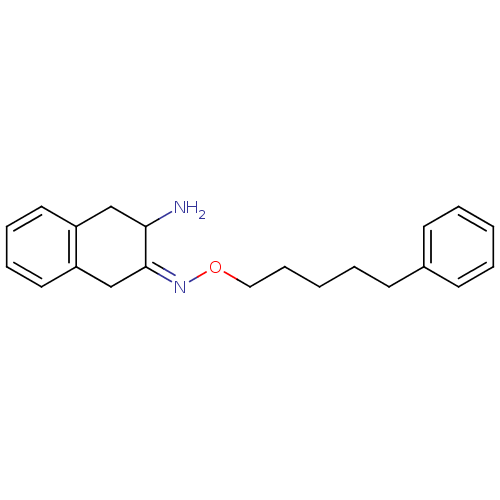
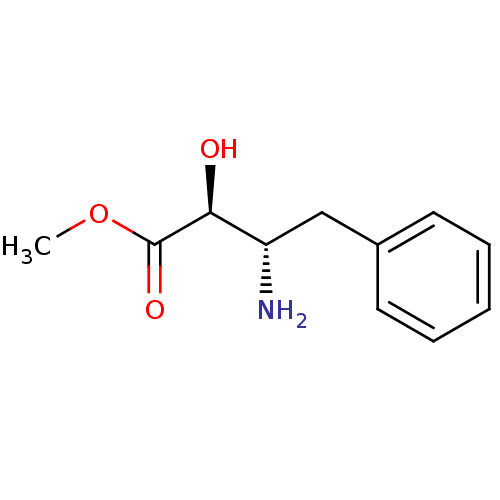
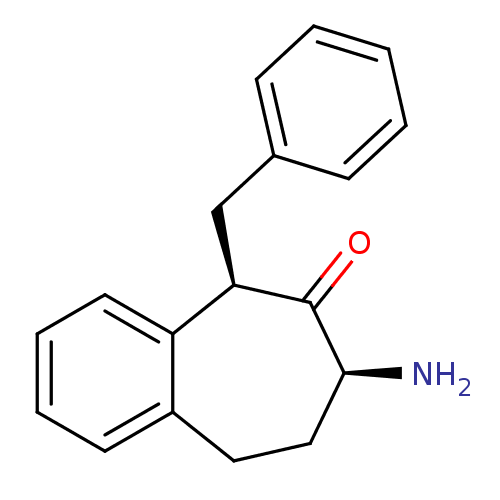
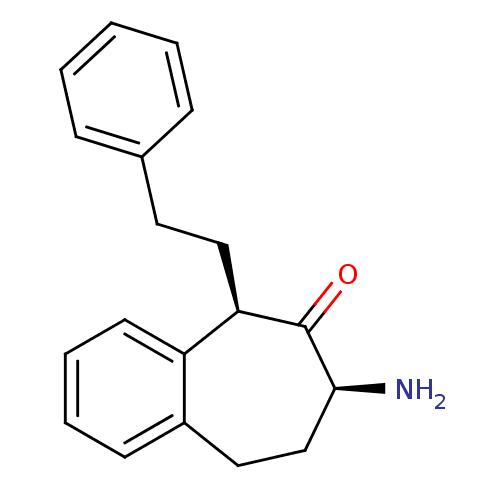
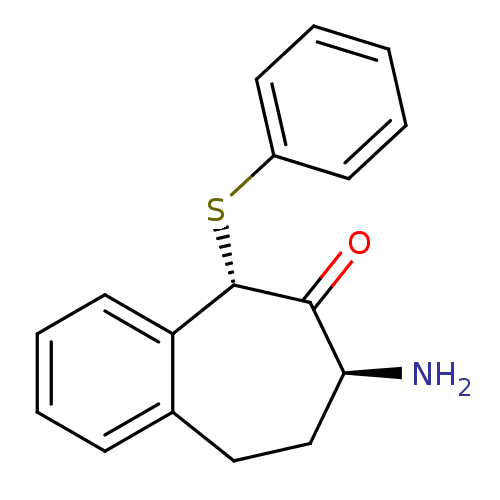
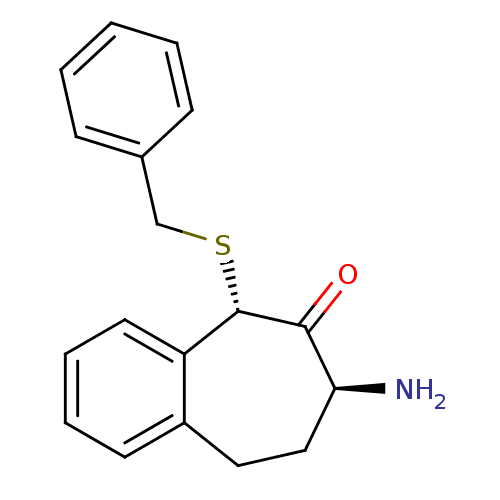
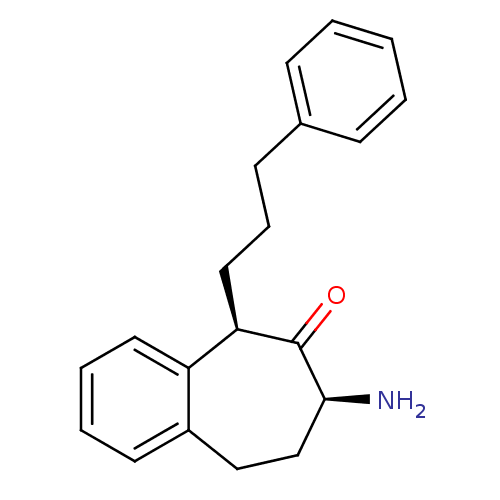
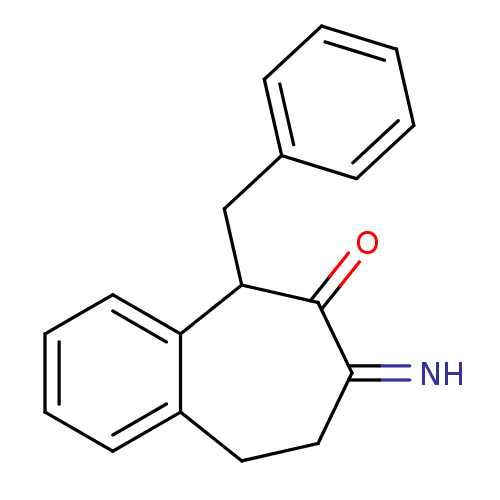
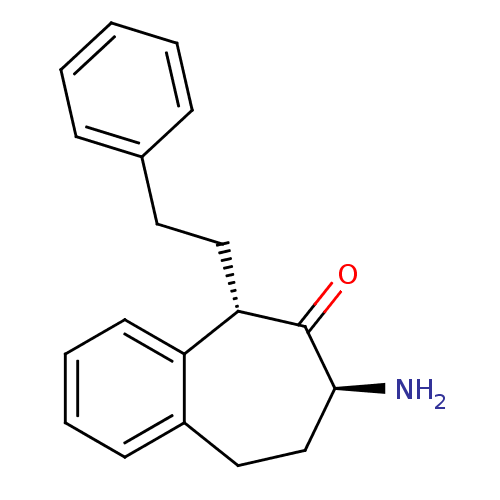
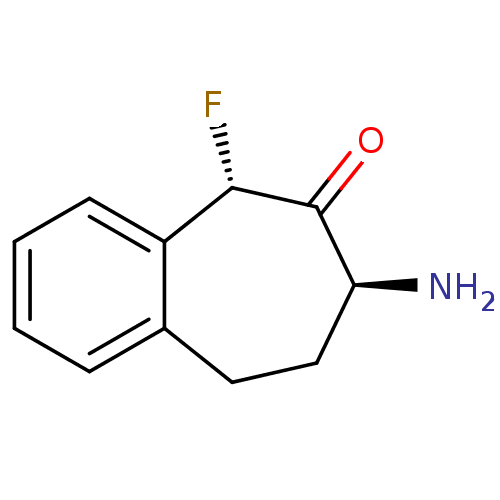
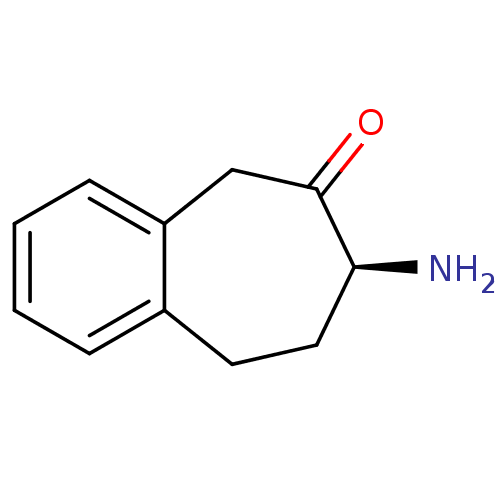
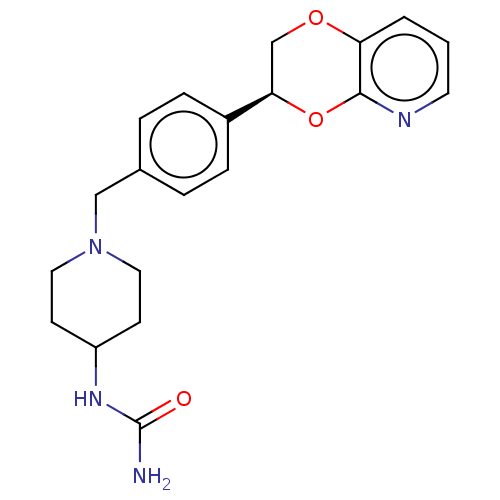
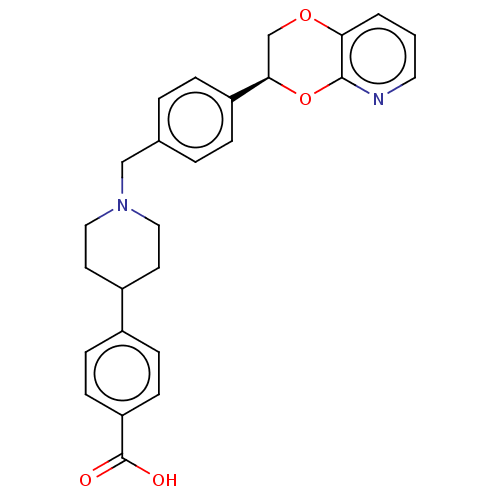
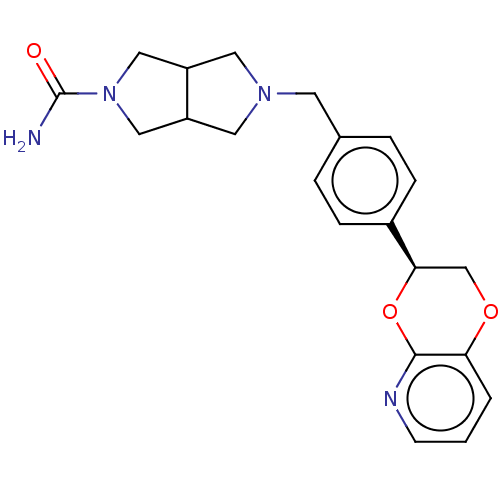
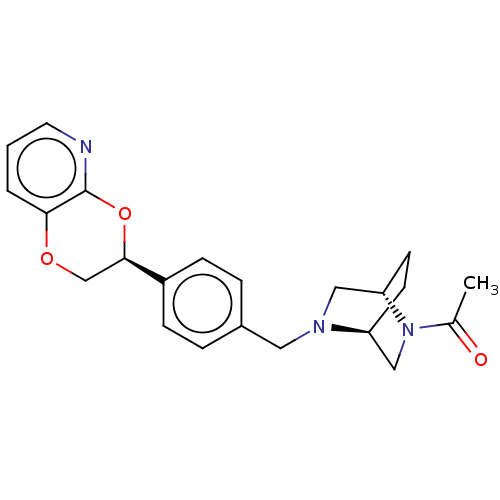
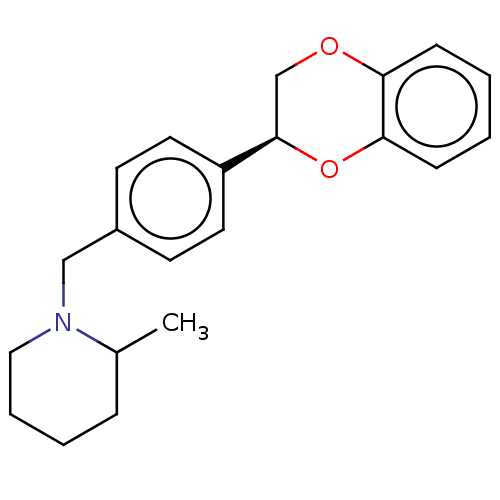
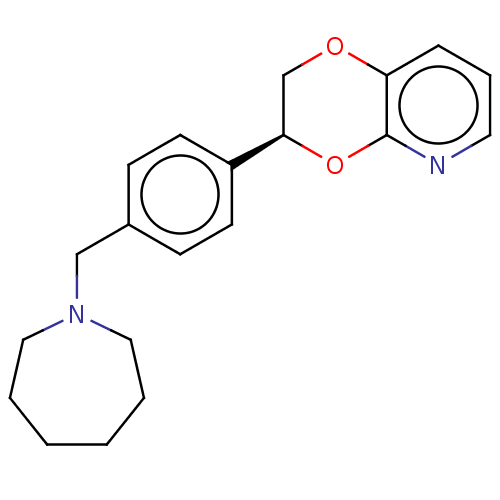
Affinity DataKi: 3nMAssay Description:Non-competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino...More data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Inhibition of human recombinant LTA4H by L-(4-benzoyl)phenylalanyl-beta-naphthylamide fluorigenic substrate by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:Inhibition of human recombinant LTA4H by L-(4-benzoyl)phenylalanyl-beta-naphthylamide fluorigenic substrate by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibitory activity against LTA 4 hydrolase in aminopeptidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitory activity against LTA 4 hydrolase in epoxide hydrolase assayMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytesMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytesMore data for this Ligand-Target Pair
Affinity DataKi: 93nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
Affinity DataKi: 172nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
Affinity DataKi: 369nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 607nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 629nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 643nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.06E+3nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.64E+3nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.59E+3nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.56E+3nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M...More data for this Ligand-Target Pair
Affinity DataKi: 4.29E+3nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t...More data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMAssay Description:Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t...More data for this Ligand-Target Pair
Affinity DataKi: 8.65E+3nMAssay Description:Inhibition of human leukotriene A-4 hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytesMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytesMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Compound was tested for inhibitory activity against Leukotriene A4 hydrolase from human leukocytesMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+4nMAssay Description:Compound was tested for inhibitory activity against Leukotriene A4 hydrolase from human leukocytesMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+4nMAssay Description:Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytesMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by Dixon-plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.70E+5nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by Dixon-plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nMAssay Description:Competitive inhibition of human recombinant LTA4H using alanine-p-nitroanilide as substrate by spectrophootmetric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0420nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ...More data for this Ligand-Target Pair

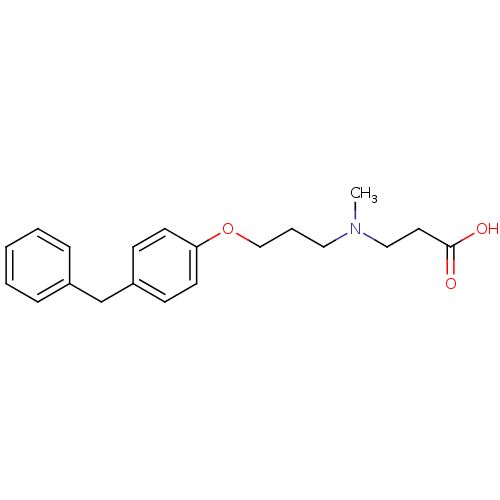
 3D Structure (crystal)
3D Structure (crystal)