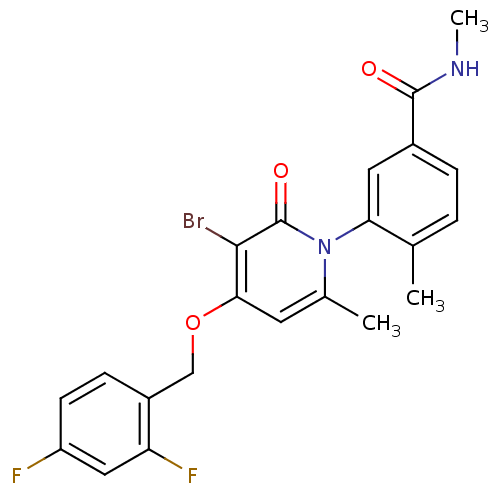
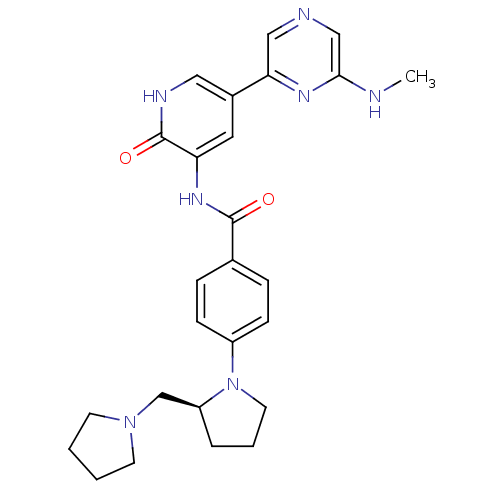
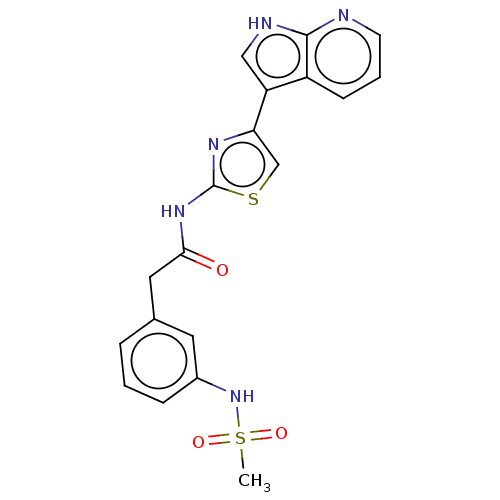
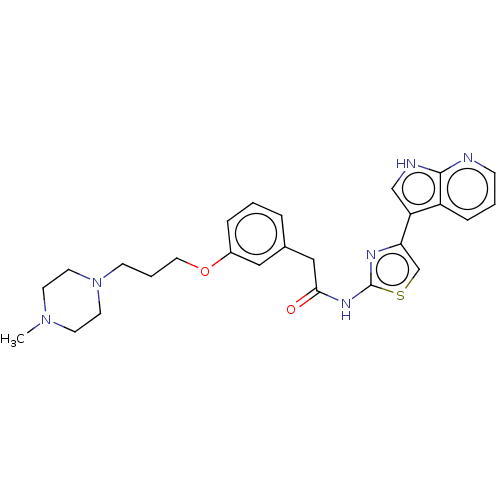
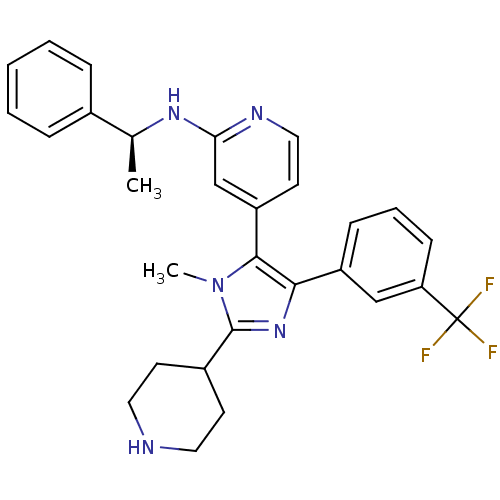
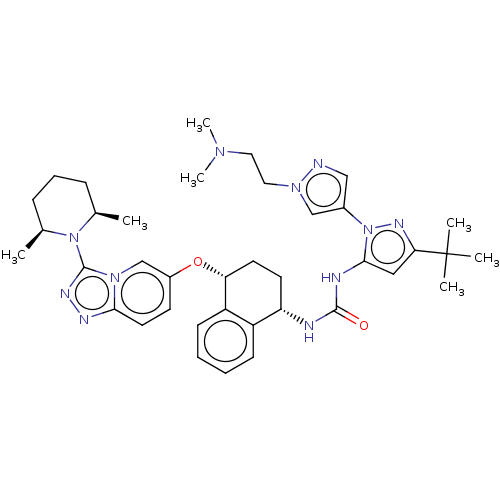
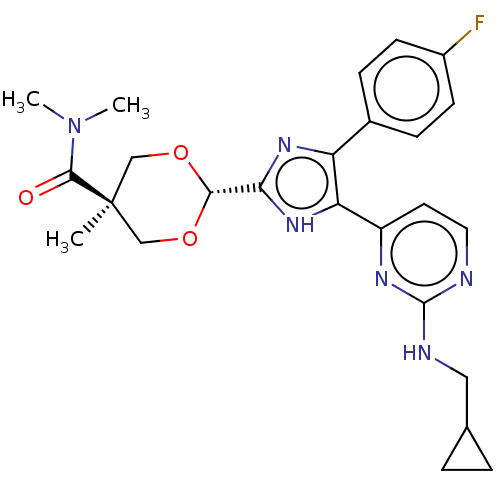
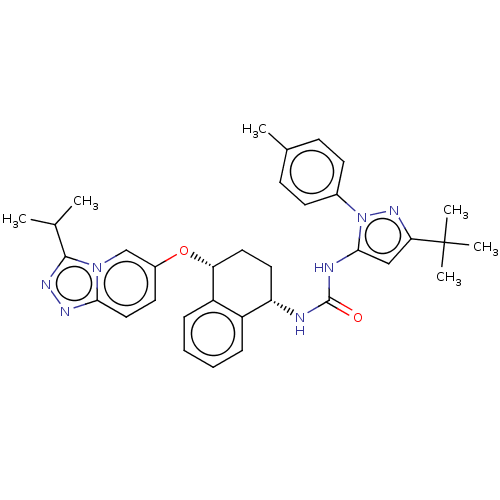
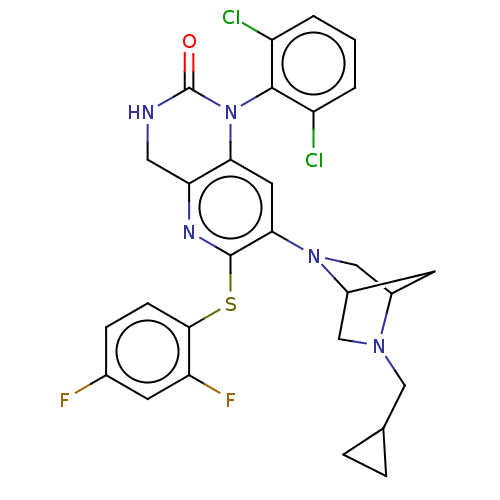
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
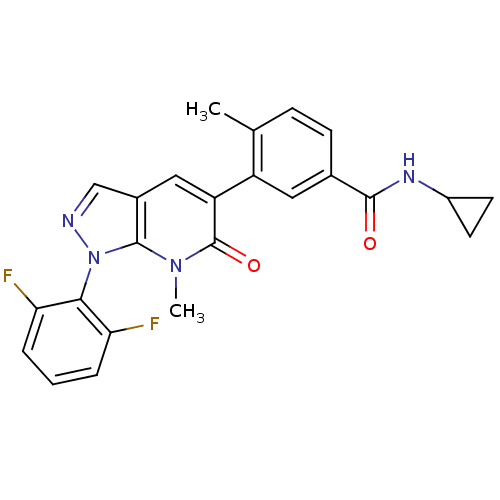
Affinity DataKi: 0.800nMAssay Description:Inhibition of P38 mitogen activated protein kinase (unknown origin)More data for this Ligand-Target Pair
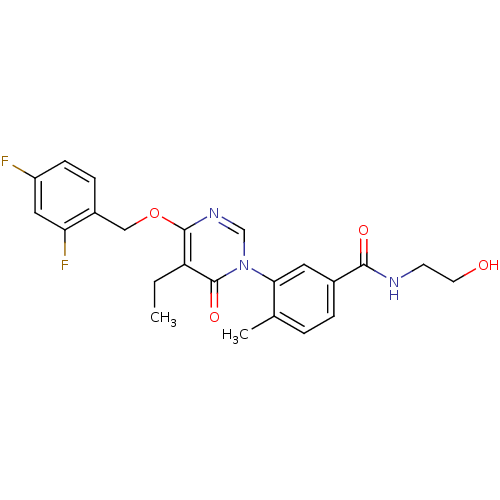
Affinity DataKi: 1.80nMAssay Description:Inhibition of human recombinant GST-tagged p38beta-induced ATF2 phosphorylationMore data for this Ligand-Target Pair
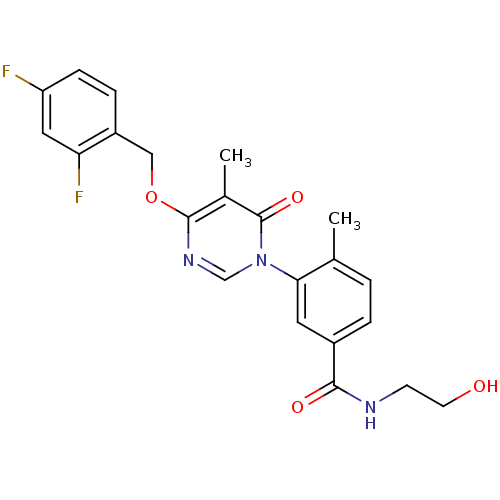
Affinity DataKi: 6nMAssay Description:Inhibition of p38beta kinaseMore data for this Ligand-Target Pair
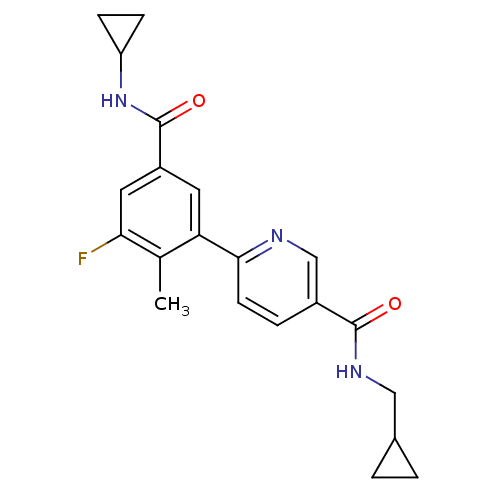
Affinity DataKi: 17nMAssay Description:Inhibition of p38beta kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny...More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Binding affinity to p38betaMore data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Inhibition of GST-tagged p38beta by fluorescence polarization methodMore data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Inhibition of GST-tagged p38beta by fluorescence polarization methodMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibition of p38beta kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 158nMAssay Description:Inhibition of GST-tagged p38beta by fluorescence polarization methodMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Inhibition of p38beta using KRELVEPLTPSGEAPNQALLR as substrate for 10 mins by lactate dehydrogenase-coupled spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Inhibitory activity against HIV-1 Y188L reverse transcriptase.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Inhibition of P38 mitogen activated protein kinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of P38betaMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of p38beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of p38beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of wild type p38beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of recombinant human full length His-tagged p38beta expressed in baculovirus expression system preincubated for 2 hrs followed by ATP addi...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Inhibition of Mitogen-activated protein kinase p38More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of Mitogen-activated protein kinase p38More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of p38-related TNF alpha release by human monocyte cell line (THP-1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant human full length His-tagged p38beta expressed in baculovirus expression system preincubated for 2 hrs followed by ATP addi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant human full length His-tagged p38beta expressed in baculovirus expression system preincubated for 2 hrs followed by ATP addi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of wild type p38beta (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of LPS-stimulated p38-related TNF-alpha production in human peripheral blood mononuclear cells (PBMC)More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human full length His-tagged p38beta expressed in baculovirus expression system preincubated for 2 hrs followed by ATP addi...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cellsMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cellsMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of Mitogen-activated protein kinase p38More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISAMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.720nMAssay Description:Inhibition of p38 MAPK-mediated TNFalpha production in LPS-induced human whole blood preincubated for 5 mins prior to LPS-treatment measured after 6 ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.740nMAssay Description:Inhibition of mitogen-activated protein kinase p38 at 2 uM ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of recombinant human full length His-tagged p38beta expressed in baculovirus expression system preincubated for 2 hrs followed by ATP addi...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of LPS-stimulated p38-related IL1-beta production in human peripheral blood mononuclear cells (PBMC)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.820nMAssay Description:Inhibition of MAPK p38 (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 0.910nMAssay Description:Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cellsMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of p38 MAP kinaseMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
Boston University
Curated by ChEMBL
Boston University
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISAMore data for this Ligand-Target Pair

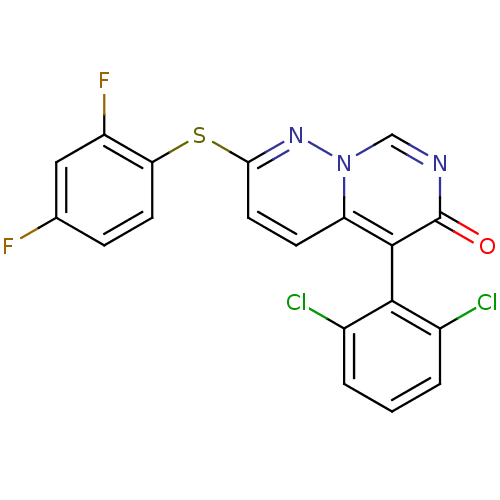
 3D Structure (crystal)
3D Structure (crystal)