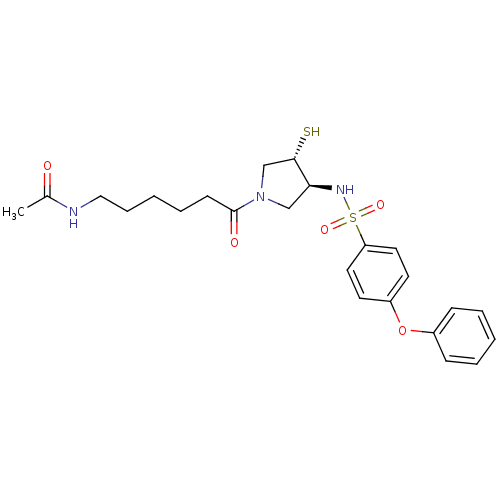
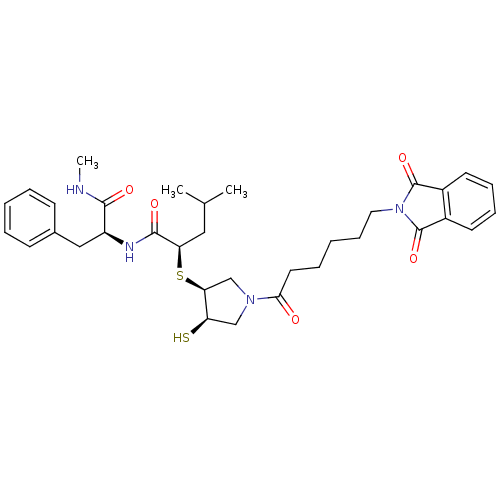
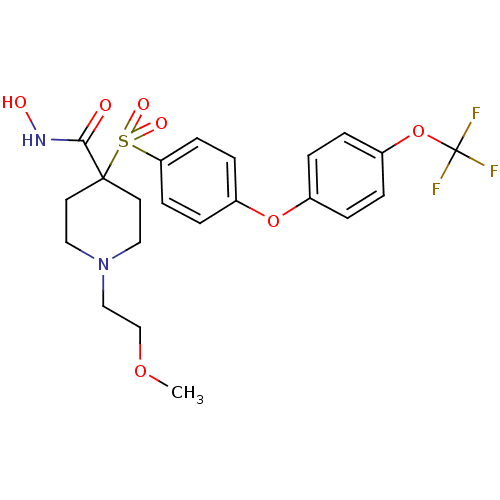
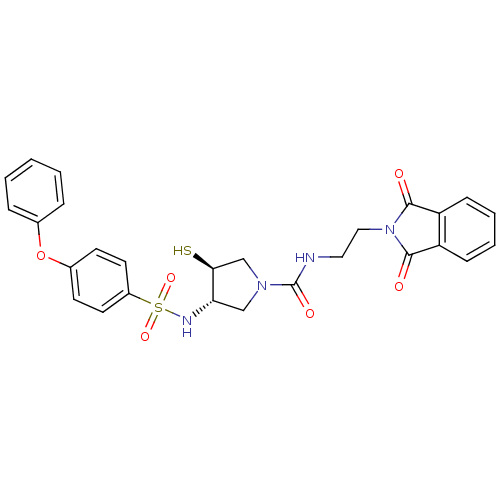
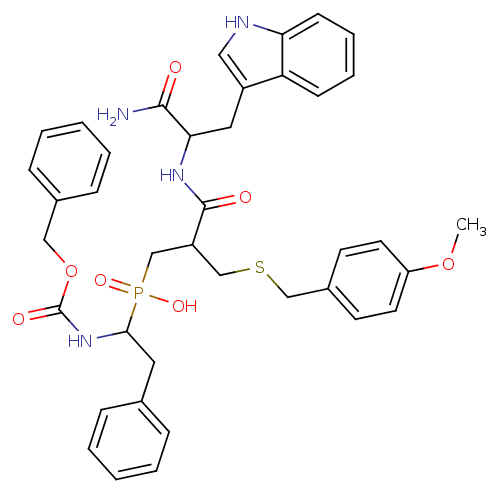
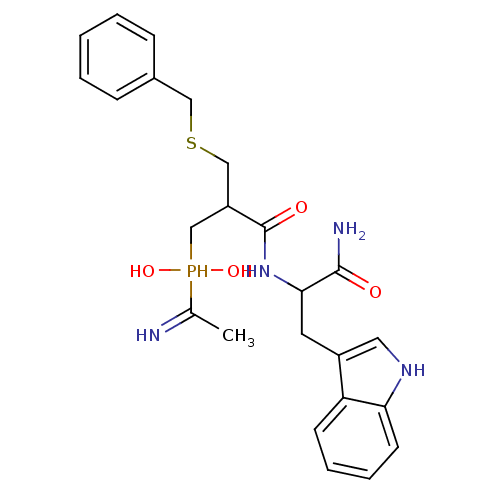
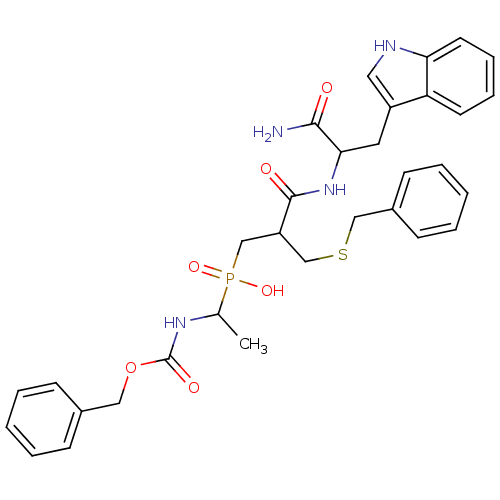
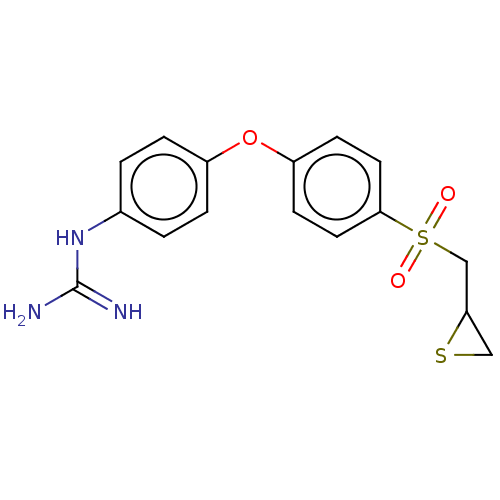
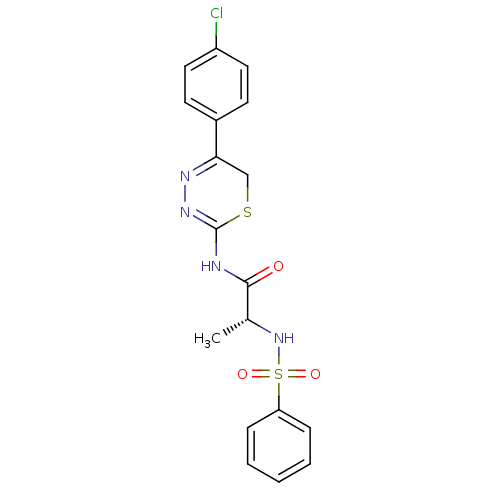
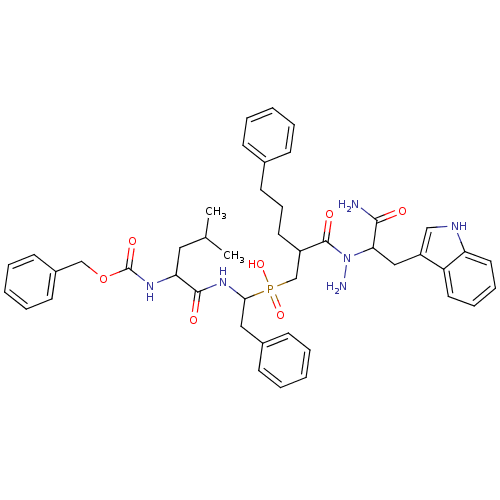
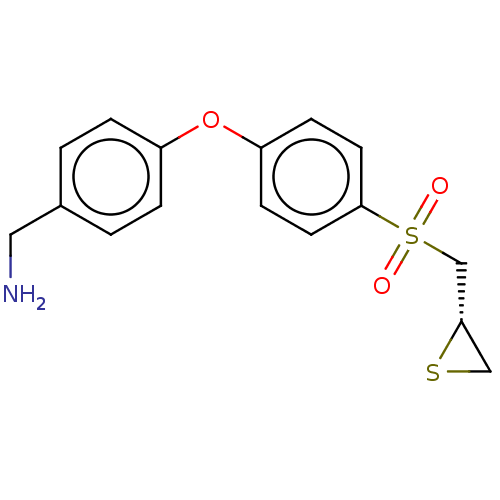
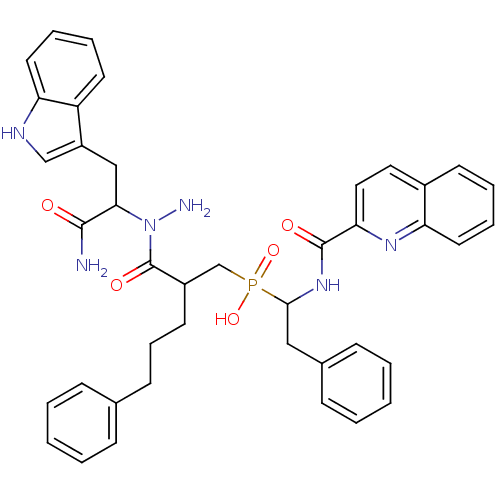
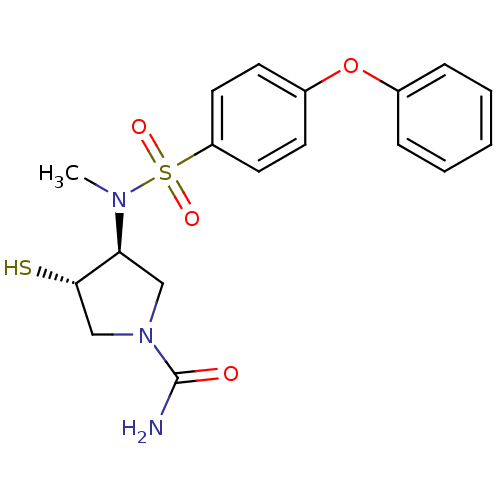
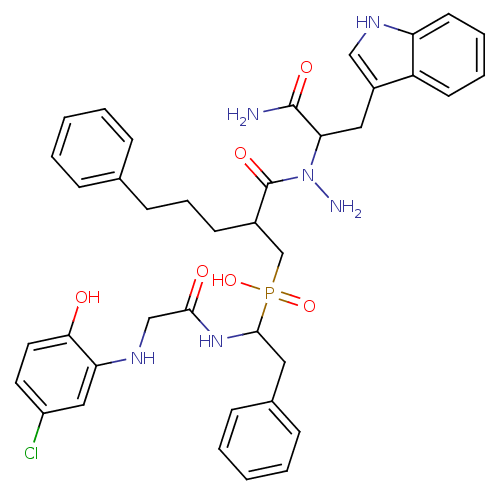
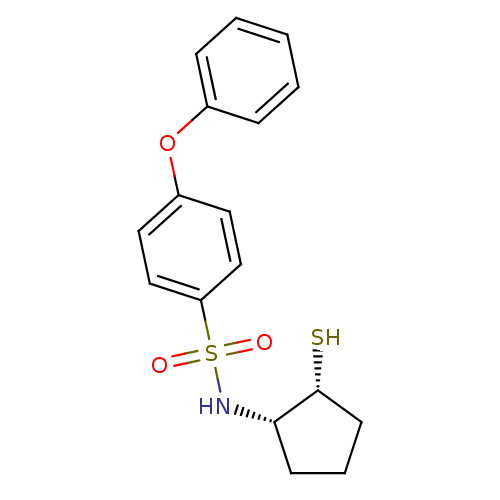
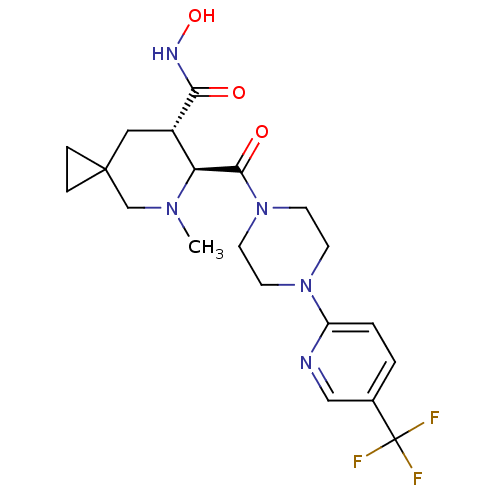
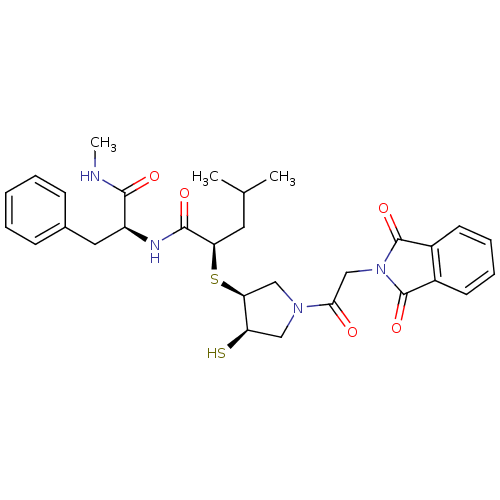
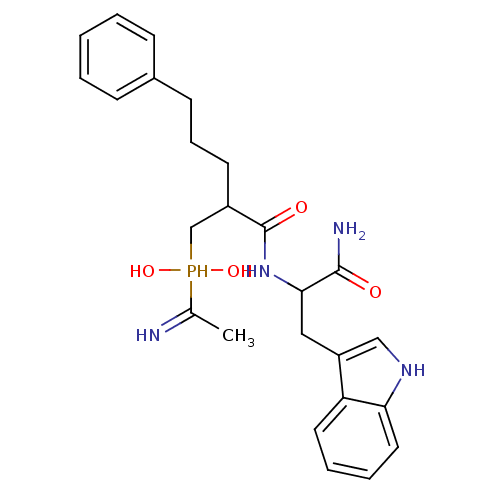
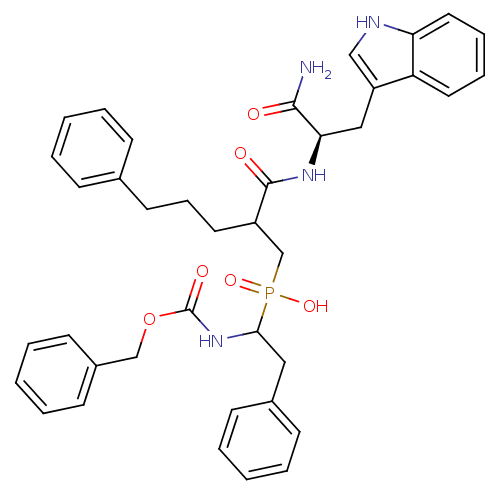
Affinity DataKi: 4nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
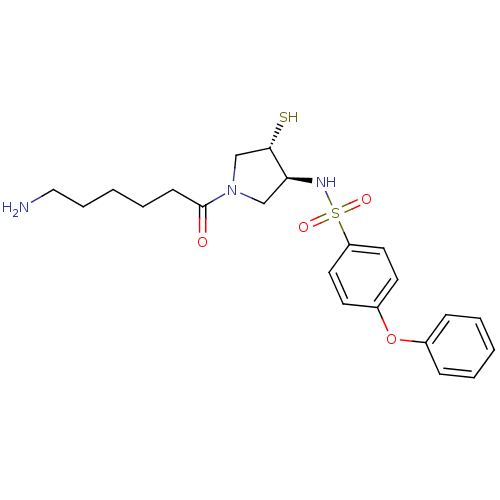
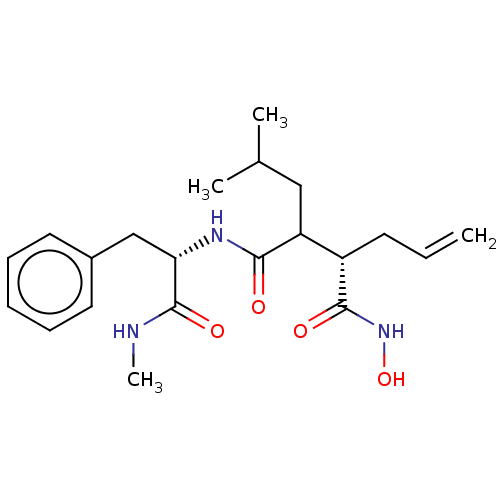
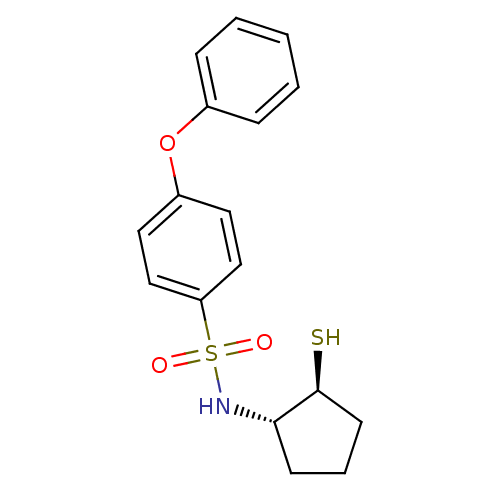
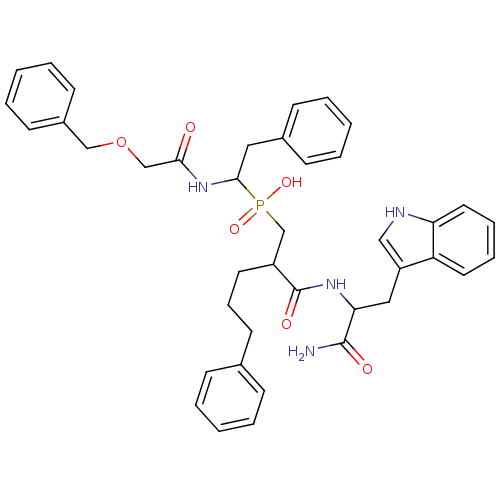
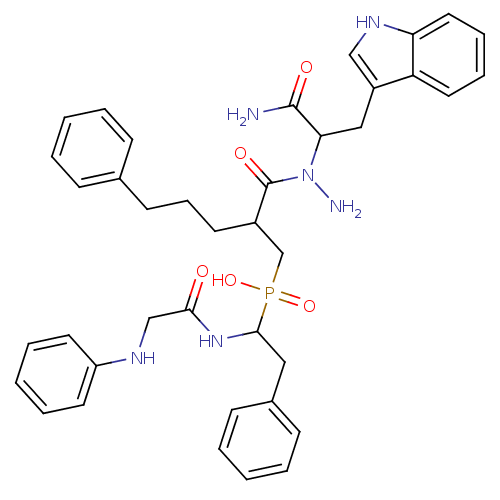
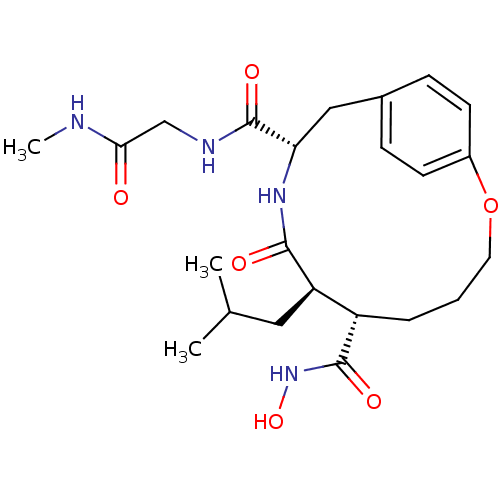
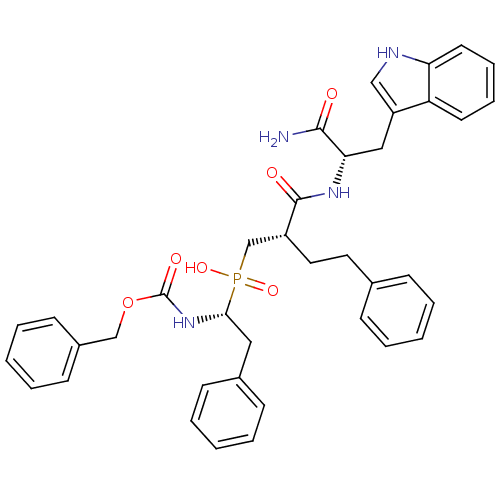
Affinity DataKi: 6nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
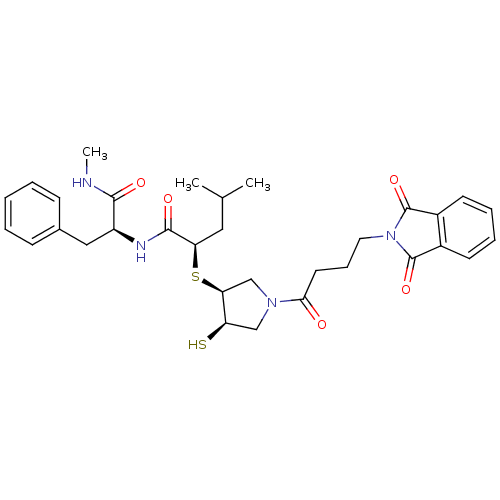
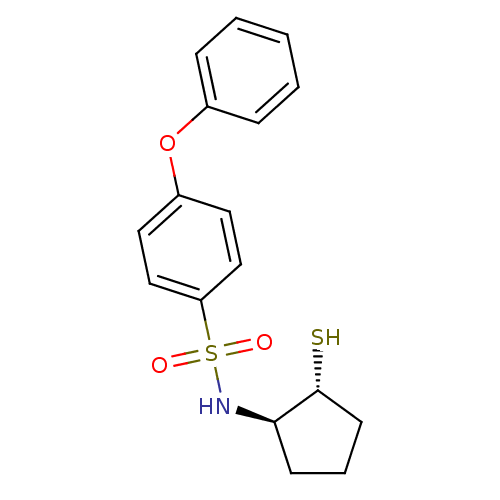
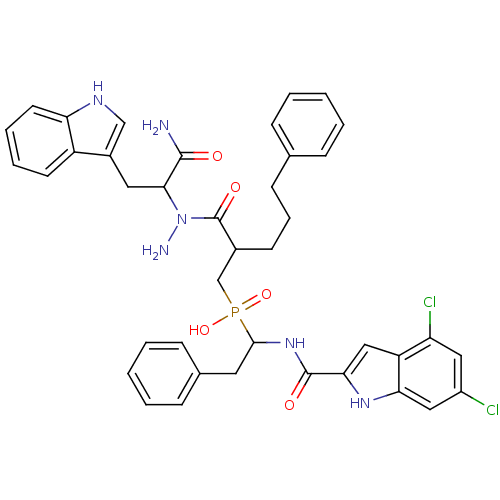
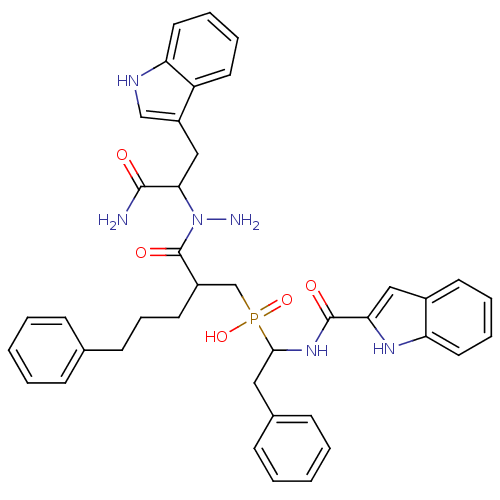
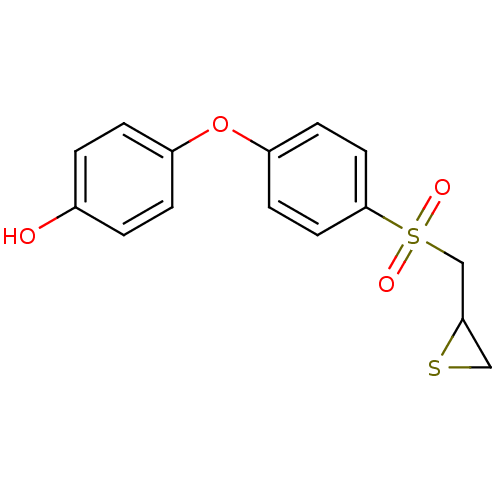
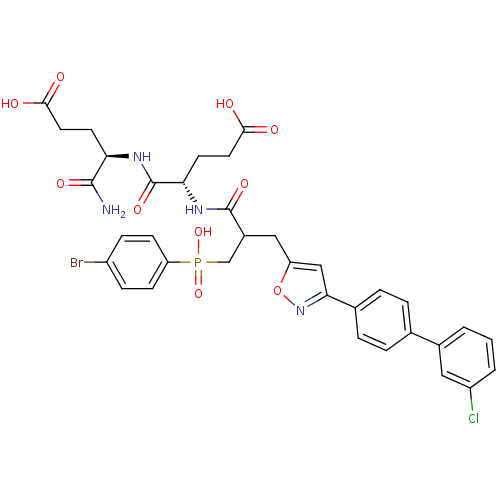
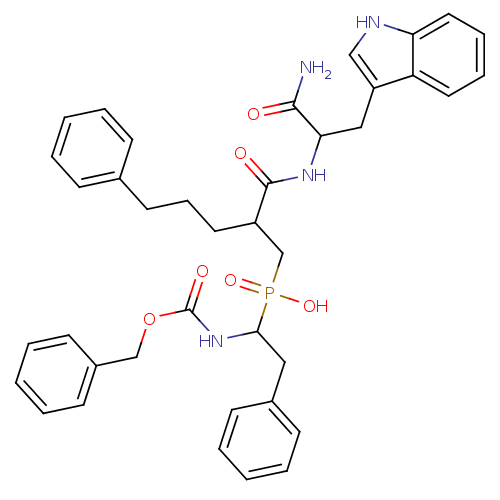
Affinity DataKi: 6.20nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
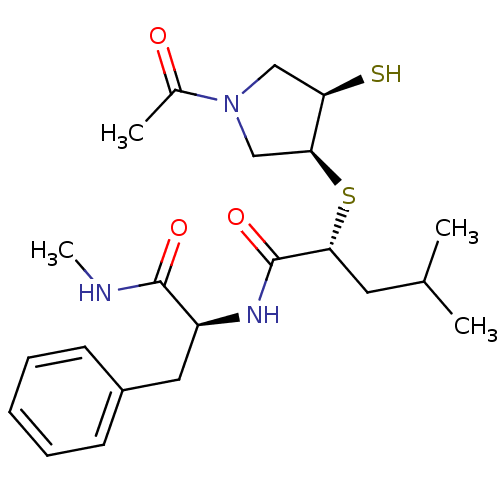
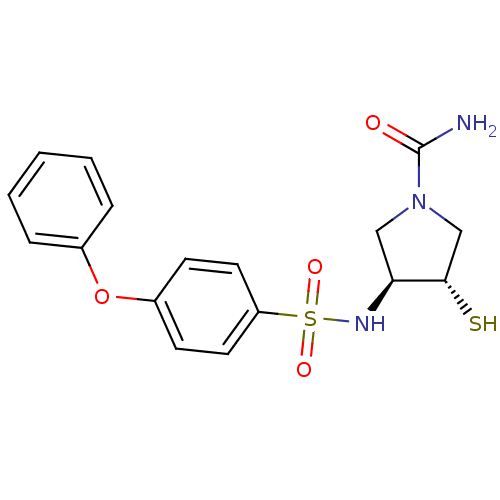
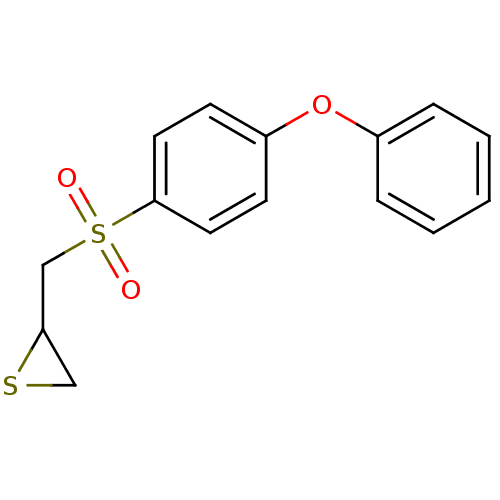
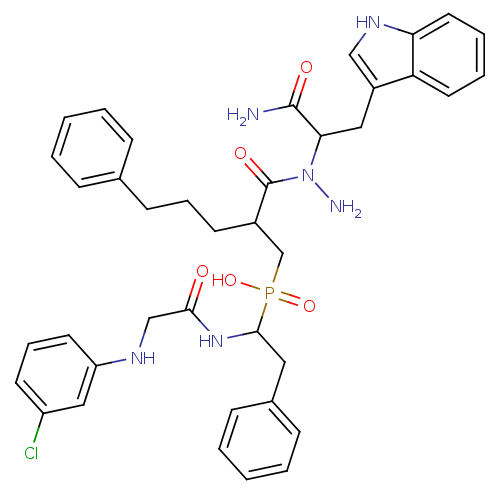
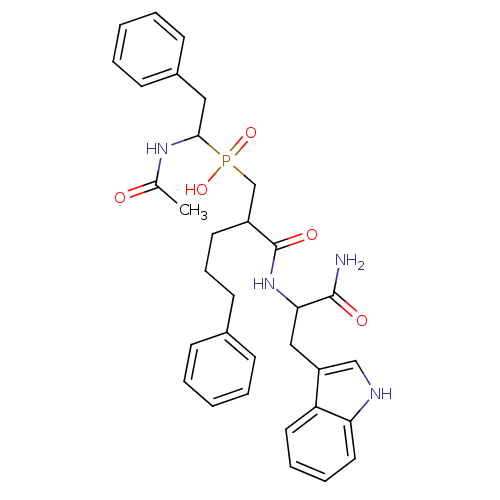
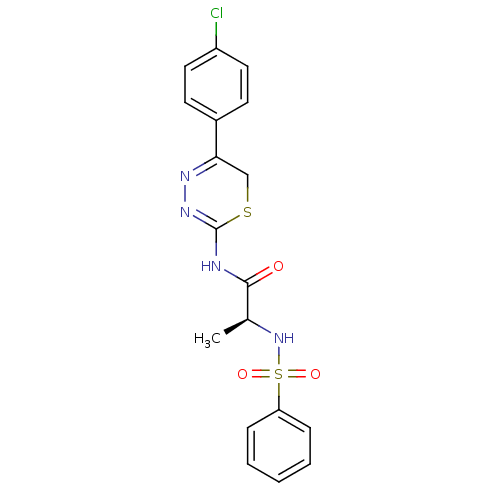
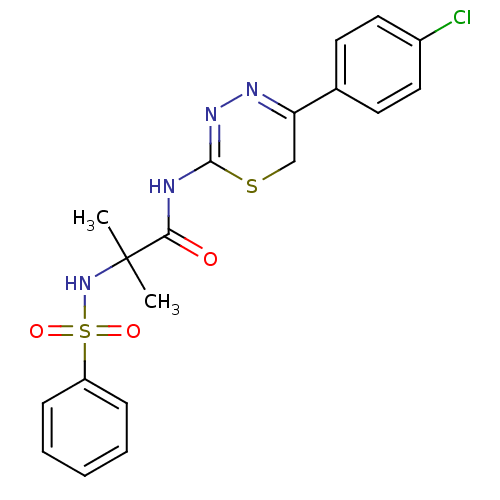
Affinity DataKi: 6.60nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of MMP-14 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of human MMP-14More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of matrix metalloproteinase-14More data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Slow binding inhibition of human recombinant MMP14 catalytic domain using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-Arg-NH2 substrateMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 68nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 68nMAssay Description:Inhibition of recombinant human MMP14 catalytic domain (89 to 265 residues) expressed in Escherichia coli using MOCAc-Pro-Leu-Gly-Leu-A2pr(Dnp)-Ala-A...More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 73nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 92nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 94nMAssay Description:Inhibition of matrix metalloprotease-14More data for this Ligand-Target Pair
Affinity DataKi: >100nM ΔG°: >-40.0kJ/molepH: 6.8 T: 2°CAssay Description:Enzyme assay using human matrix metalloproteases or ADAMTS.More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:In vitro inhibitory activity against the catalytic domain of human matrix metalloprotease-14More data for this Ligand-Target Pair
Affinity DataKi: 105nMAssay Description:Binding affinity against matrix metalloprotease-14 MTI-MMP.More data for this Ligand-Target Pair
Affinity DataKi: 105nMAssay Description:Inhibition of Matrix metalloprotease-14More data for this Ligand-Target Pair
Affinity DataKi: 105nMAssay Description:Binding affinity to MMP14More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Inhibition of human recombinant MMP14 catalytic domain by substrate hydrolysis based fluorescence spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Inhibition of MMP-14 (unknown origin)More data for this Ligand-Target Pair
TargetMatrix metalloproteinase-14 [1-20,P8S](Homo sapiens (Human))
Commissariat A L'Energie Atomique Et Aux Energies Alternatives
US Patent
Commissariat A L'Energie Atomique Et Aux Energies Alternatives
US Patent
Affinity DataKi: 118nMAssay Description:The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ...More data for this Ligand-Target Pair