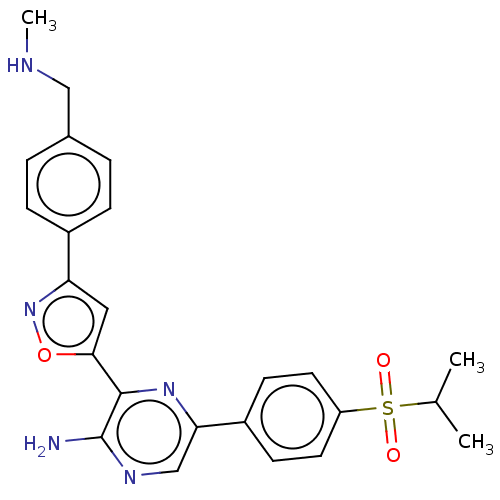
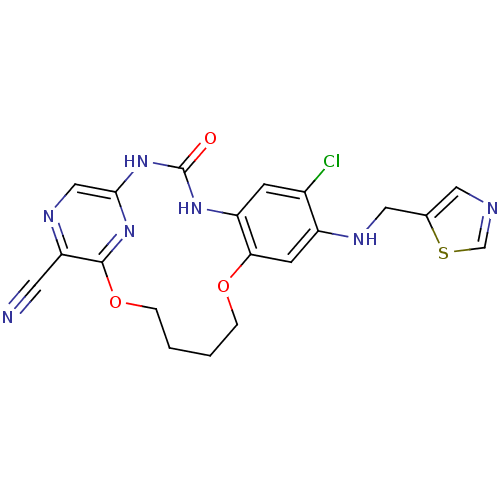
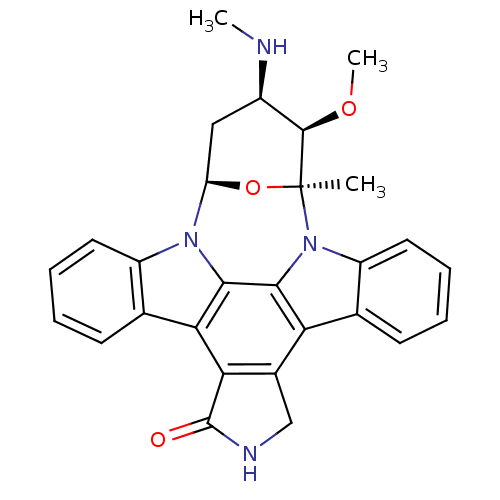
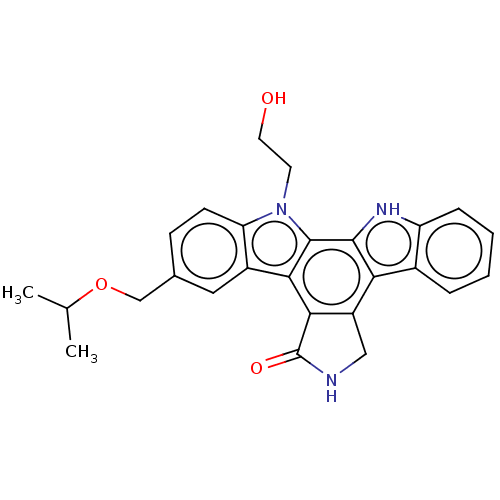
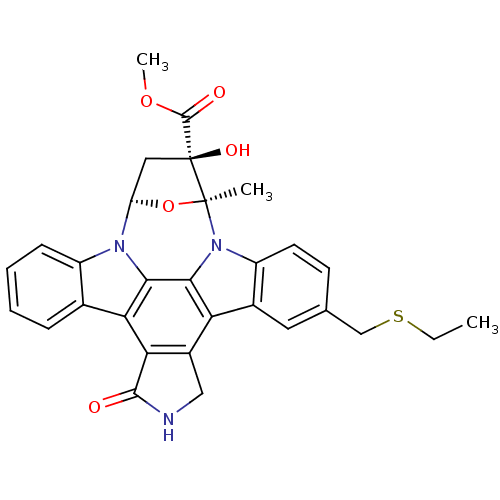
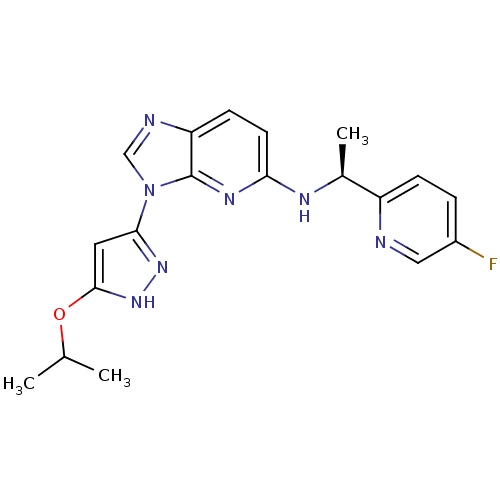
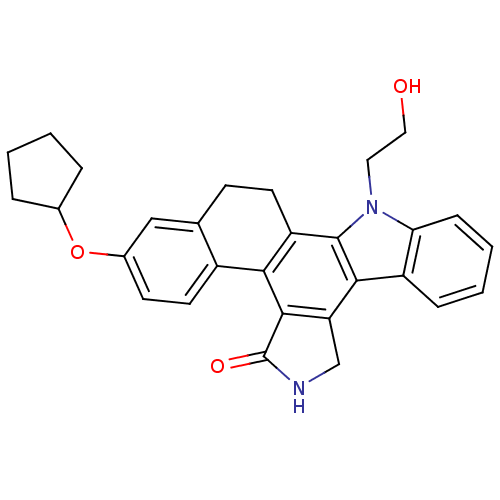
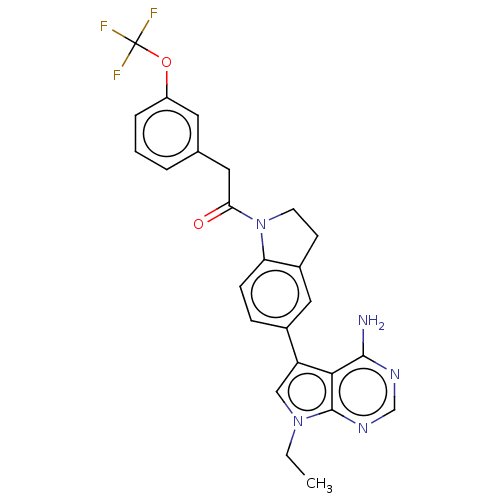
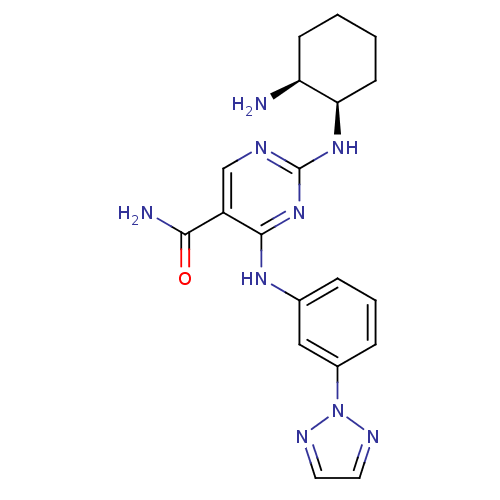
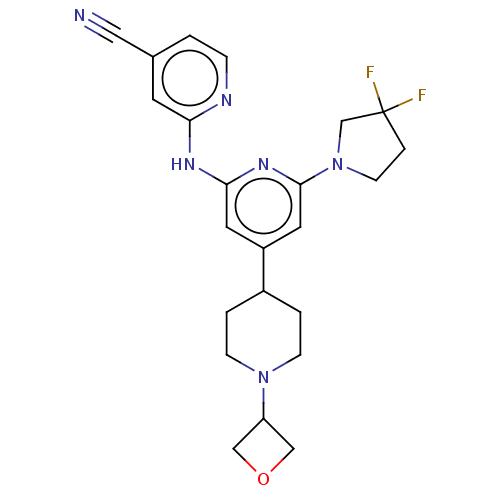
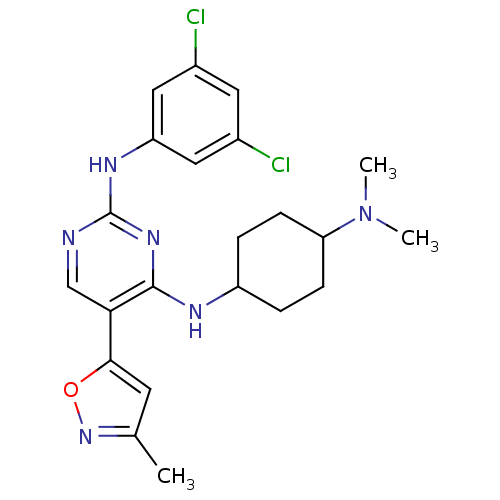
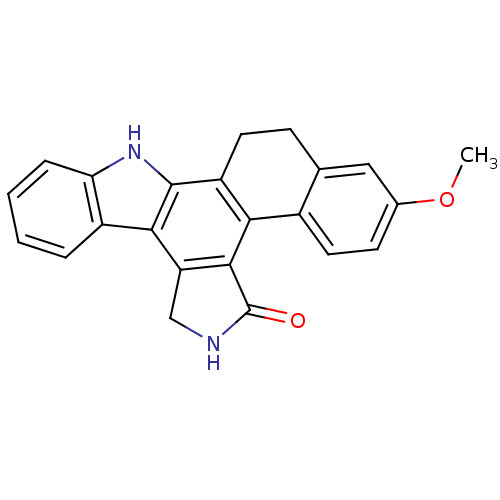
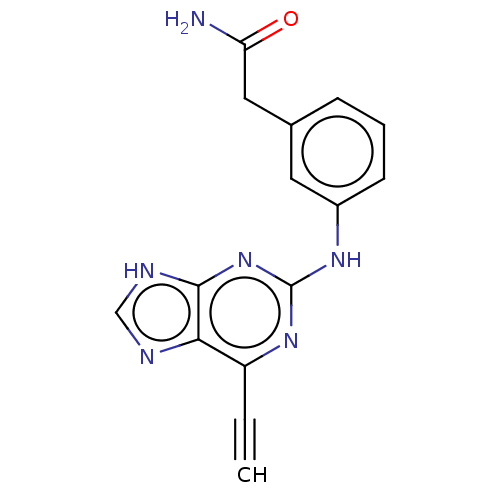
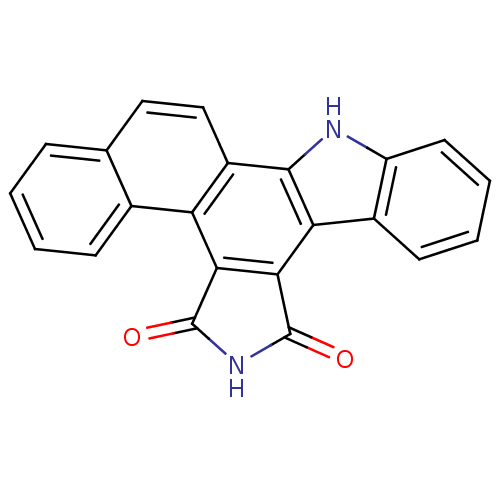
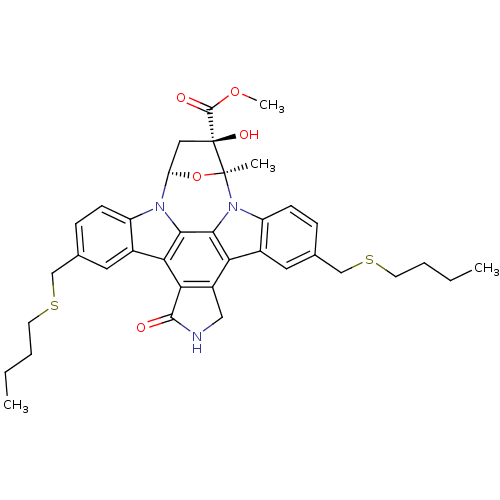
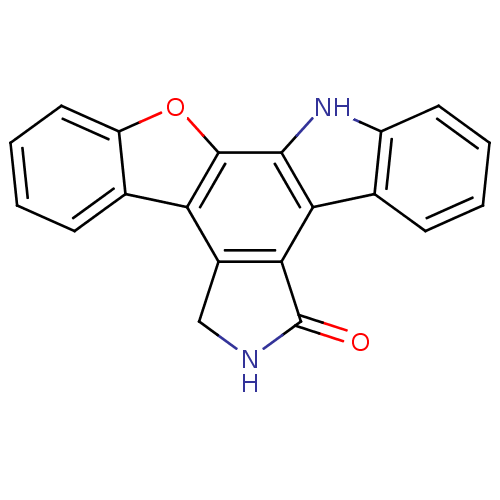
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:ATP competitive inhibition of human MLK1More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataKi: >1.67E+3nMAssay Description:Inhibition of MLK1More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 0.544nMAssay Description:Inhibition of human MLK1 using casein as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human MLK1 using casein as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of MLK1 (unknown origin) by NanoBRET cellular target engagement assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant GST-tagged human MLK1 KD (1 to 500 residues) expressed in baculovirus using myelin basic protein as substrate preincubated ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of MLK1 (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 22nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 22nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 26nMpH: 7.2 T: 2°CAssay Description:The MLK kinase assays were performed by using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. The inhibition curves were genera...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 38nMpH: 7.2 T: 2°CAssay Description:The MLK kinase assays were performed by using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. The inhibition curves were genera...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of recombinant GST-tagged human MLK1 KD (1 to 500 residues) expressed in baculovirus using myelin basic protein as substrate preincubated ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 46nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 49nMpH: 7.2 T: 2°CAssay Description:The MLK kinase assays were performed by using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. The inhibition curves were genera...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:Inhibition of MLK1 (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 52nMAssay Description:Inhibition of recombinant human MLK1 (134 to 414 residues) using casein as substrate after 40 mins in presence of [gamma-33P]-ATP by scintillation co...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Employing the Milipore panel of purified kinases EXAMPLE 87 (IC50=1 nM) inhibited 98% of purified Syk kinase activity at 50 nM. IC50 values were dete...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 66nMpH: 7.2 T: 2°CAssay Description:The MLK kinase assays were performed by using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. The inhibition curves were genera...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 68nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 68nMAssay Description:Inhibition of MLK1 (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 69nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 101nMpH: 7.2 T: 2°CAssay Description:The MLK kinase assays were performed by using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. The inhibition curves were genera...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 103nMpH: 7.2 T: 2°CAssay Description:The MLK kinase assays were performed by using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. The inhibition curves were genera...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 111nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 138nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of MAP3K9 (unknown origin) in presence of ATPMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 162nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 167nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 188nMpH: 7.2 T: 2°CAssay Description:The MLK kinase assays were performed by using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. The inhibition curves were genera...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of human MLK1 by kinase-profiling analysisMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 300nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: >300nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 303nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 308nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 360nMAssay Description:Inhibition of Mixed lineage kinase 1 (MLK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Inhibition of MLK1 (unknown origin) using [gamma-33P]ATPMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 482nMpH: 7.2 T: 2°CAssay Description:The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
TargetMitogen-activated protein kinase kinase kinase 9(Homo sapiens (Human))
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Vertex Pharmaceuticals (Europe)
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of MLK1 (unknown origin) by [33P]-ATP filter binding kinase assayMore data for this Ligand-Target Pair