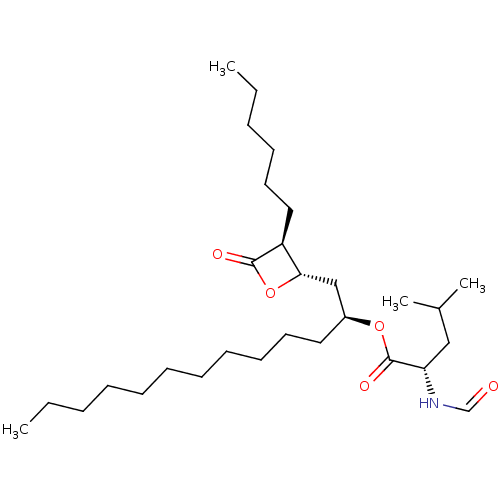
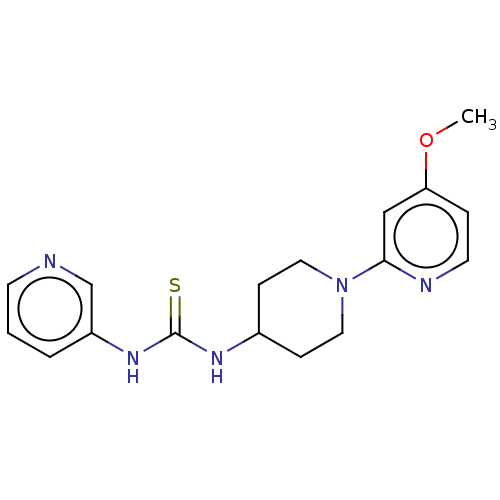
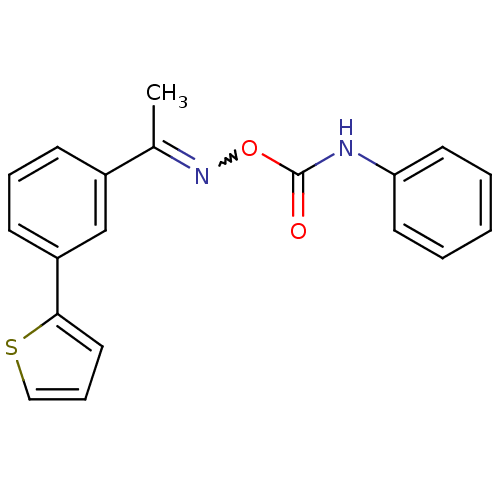
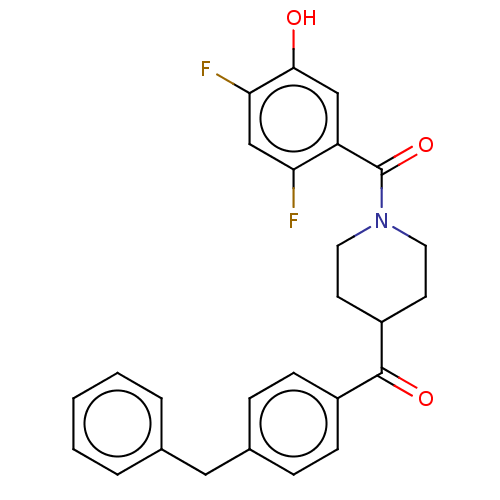
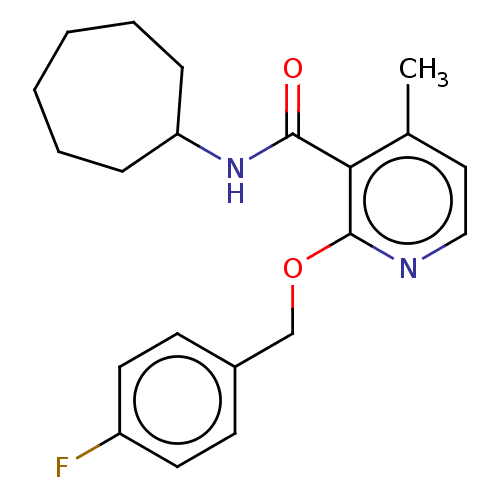
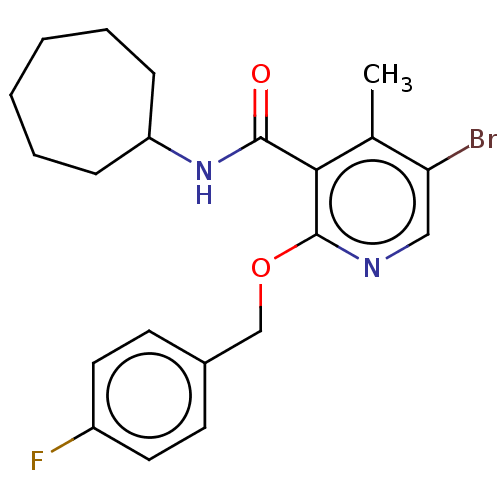
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
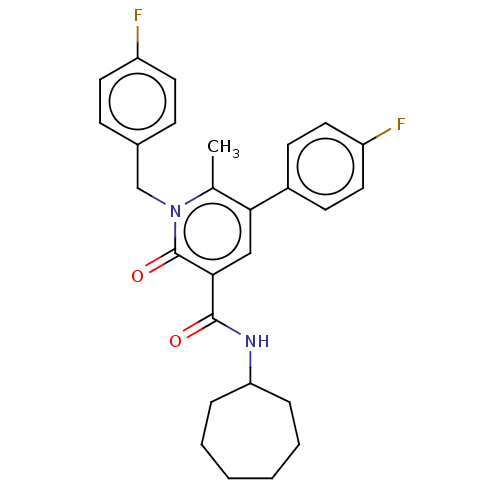
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
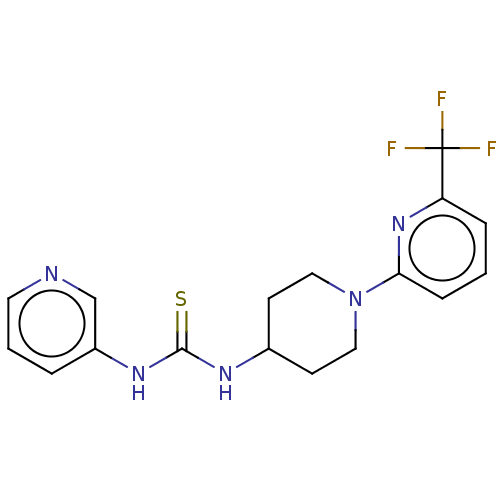
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
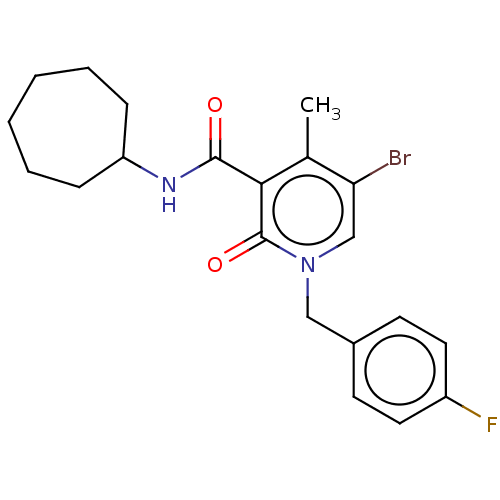
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant ABHD12 transfected in HEK293T cells by SDS-PAGE using rhodamine-tagged FP probeMore data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 89nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 96nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human ABHD12 containing pCMV6-XL4-hABHD12 transfected into HEK293 cellsMore data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells pre-incubated for 10 mins before 2-AG substrate addition by HPLC methodMore data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells pre-incubated for 10 mins before 2-AG substrate addition by HPLC methodMore data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of FP-Rh probe binding to ABHD12 in mouse brain membrane proteome incubated for 45 mins by CuAAC fluorescence dependent gel-based competit...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of FP-Rh probe binding to ABHD12 in mouse brain membrane proteome incubated for 45 mins by CuAAC fluorescence dependent gel-based competit...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 410nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 630nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 640nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of JJH350 binding to ABHD12 in mouse brain membrane proteome incubated for 20 mins by CuAAC fluorescence dependent gel-based competitive A...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Mus musculus)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of mouse brain MAGLMore data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft...More data for this Ligand-Target Pair
TargetLysophosphatidylserine lipase ABHD12(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of recombinant human ABHD12 expressed in HEK293 cells using 2-OG as substrate pretreated for 30 mins followed by substrate addition and me...More data for this Ligand-Target Pair