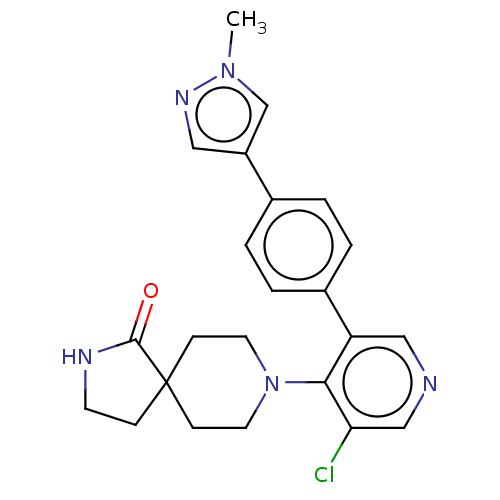
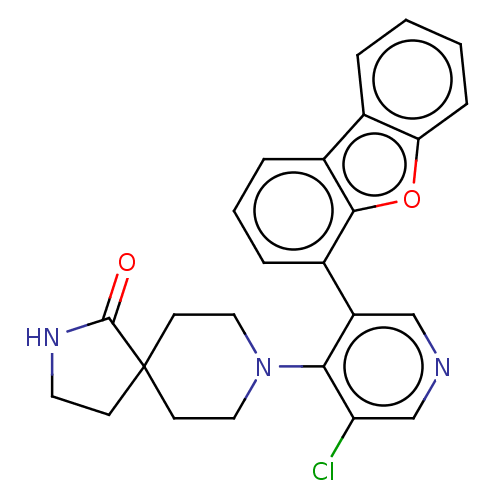
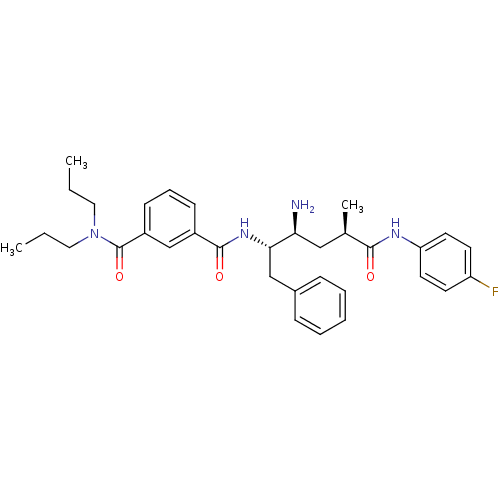
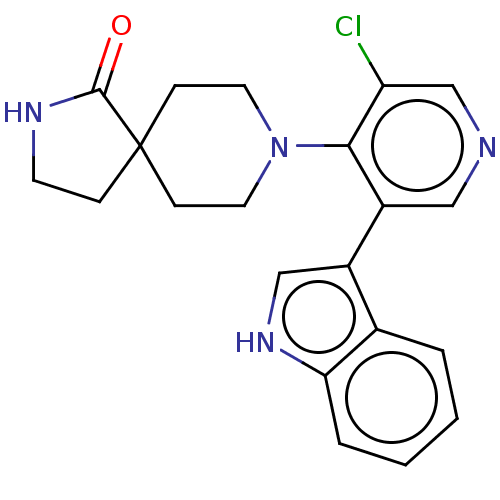
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
University Of Kentucky
Curated by ChEMBL
University Of Kentucky
Curated by ChEMBL
Affinity DataKi: 0.170nMAssay Description:Inhibition of human recombinant FLT3 by radiometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.324nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.417nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Competitive kinetic for thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.537nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.813nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.813nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.933nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.41nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.58nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.86nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.82nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.39nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:In vitro reversible inhibition of thrombin catalytic activityMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of South Australia
Curated by ChEMBL
University Of South Australia
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of human GSKbeta H350L mutant (2 to 3nd residues) using YRRAAVPPSPSLSRHSSPHQS(p) EDEEE as substrate incubated for 40 mins in presence of [...More data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Concentration of the compound required to inhibit Human alpha-thrombin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Competitive kinetic for human alpha thrombin inhibition Ki was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 alpha(Homo sapiens (Human))
University Of South Australia
Curated by ChEMBL
University Of South Australia
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibition of human GSKalpha S449A mutant (2 to 3nd residues) using YRRAAVPPSPSLSRHSSPHQS(p) EDEEE as substrate incubated for 40 mins in presence of ...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of South Australia
Curated by ChEMBL
University Of South Australia
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Inhibition of human GSKbeta H350L mutant (2 to 3nd residues) using YRRAAVPPSPSLSRHSSPHQS(p) EDEEE as substrate incubated for 40 mins in presence of [...More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 22.4nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -42.9kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 26.3nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 33nM ΔG°: -42.3kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
University Of Kentucky
Curated by ChEMBL
University Of Kentucky
Curated by ChEMBL
Affinity DataKi: 42nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 alpha(Homo sapiens (Human))
University Of South Australia
Curated by ChEMBL
University Of South Australia
Curated by ChEMBL
Affinity DataKi: 43nMAssay Description:Inhibition of human GSKalpha S449A mutant (2 to 3nd residues) using YRRAAVPPSPSLSRHSSPHQS(p) EDEEE as substrate incubated for 40 mins in presence of ...More data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 58nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
University Of South Australia
Curated by ChEMBL
University Of South Australia
Curated by ChEMBL
Affinity DataKi: 61nMAssay Description:Inhibition of human full length PKCtheta using histone H1 as substrate incubated for 40 mins in presence of [gamma-33P]-ATP by scintillation counting...More data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Inhibition of CDK8/cyclin C (unknown origin) using RBER-IRStide as substrate incubated for 60 mins in presence of [gamma33P]ATP by scintillation coun...More data for this Ligand-Target Pair
Affinity DataKi: 71nM ΔG°: -40.4kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair


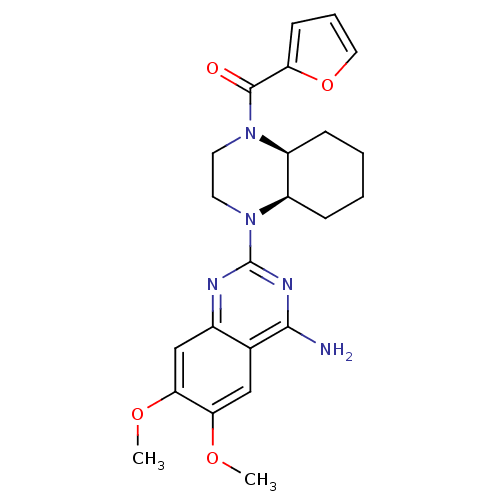
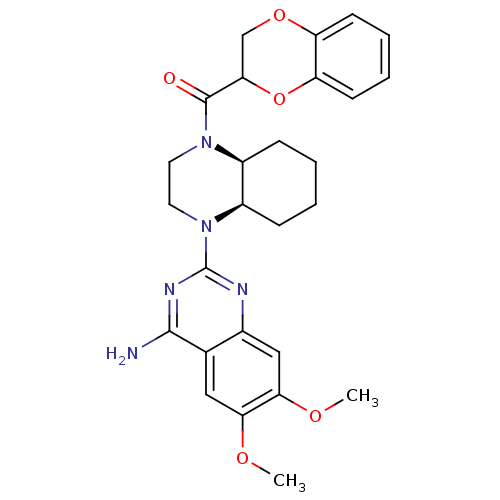
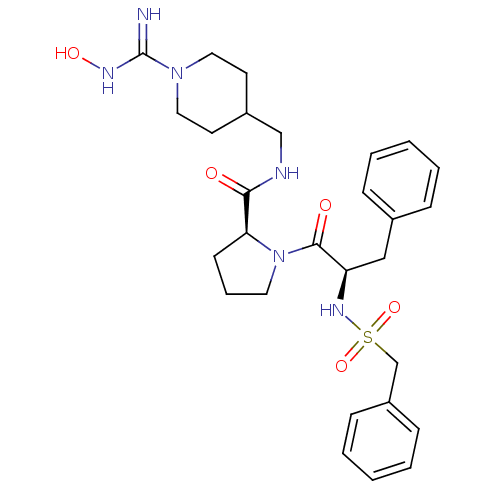

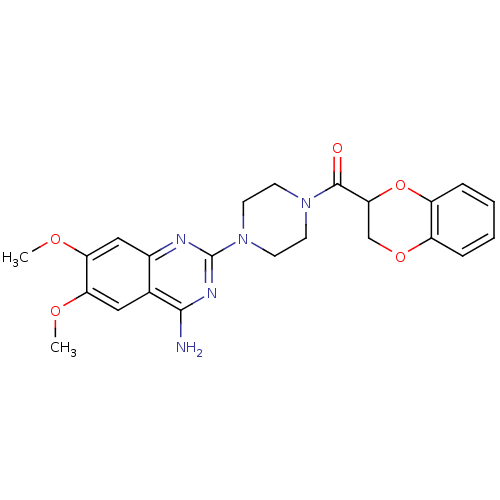

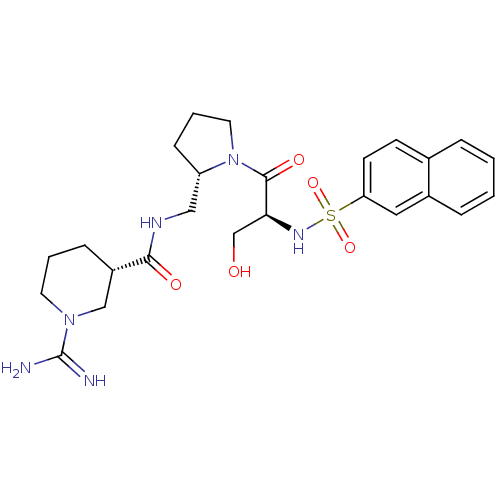
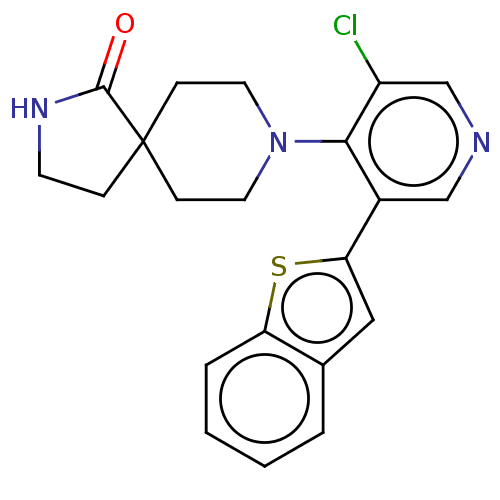
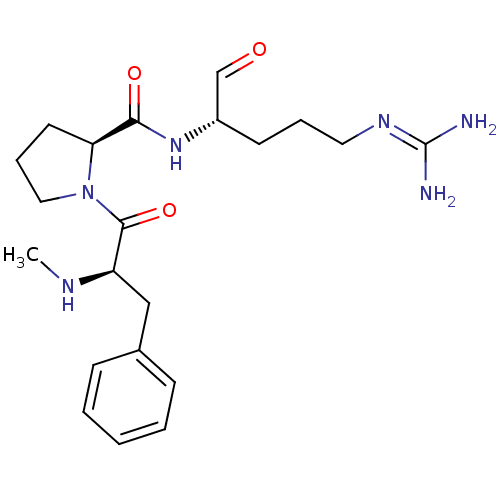
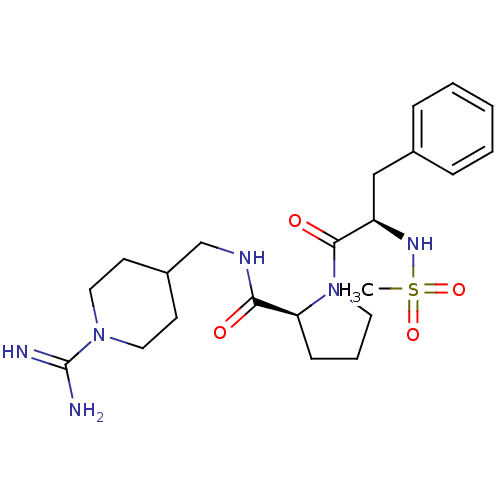
 3D Structure (crystal)
3D Structure (crystal)