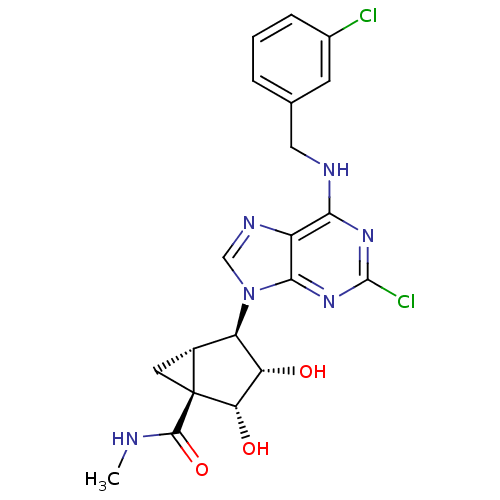
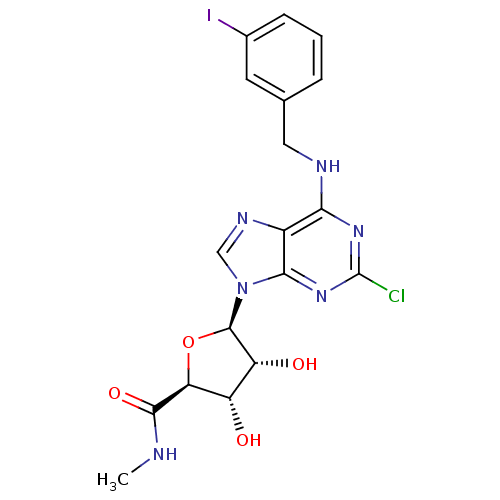
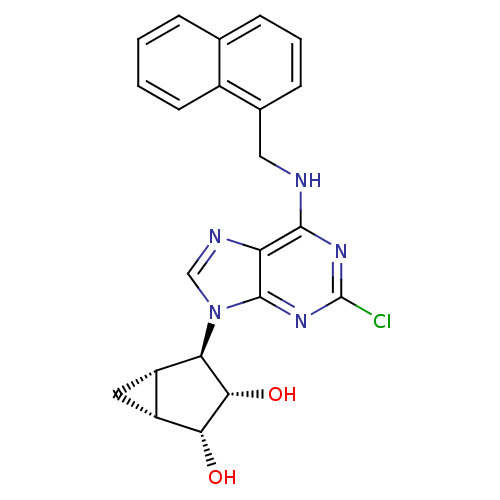
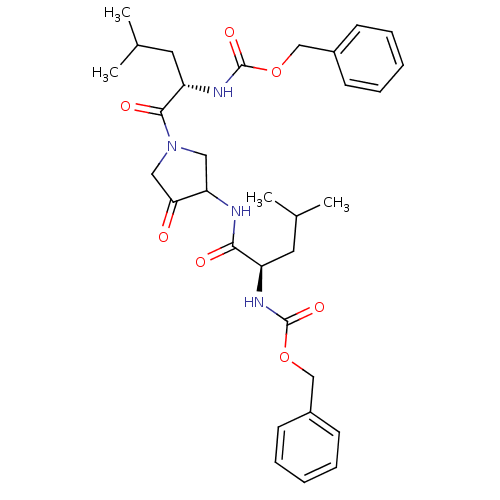
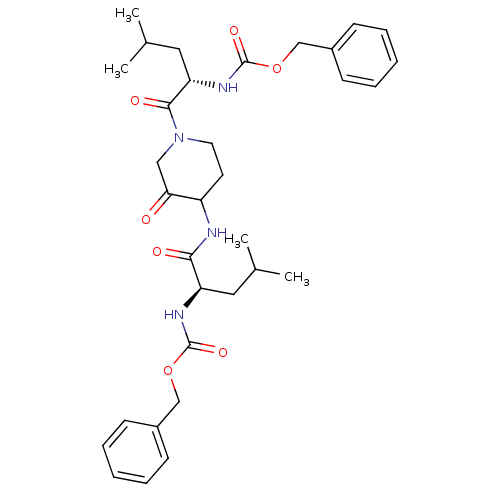
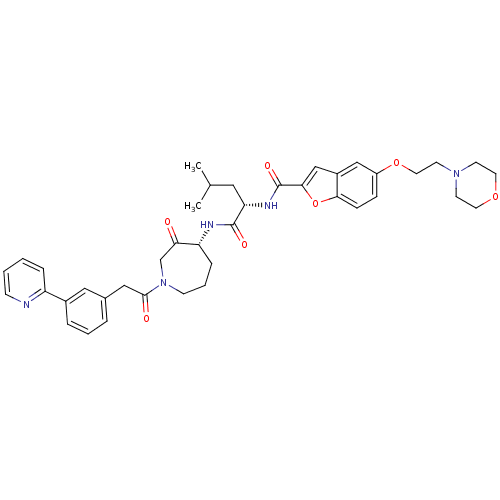
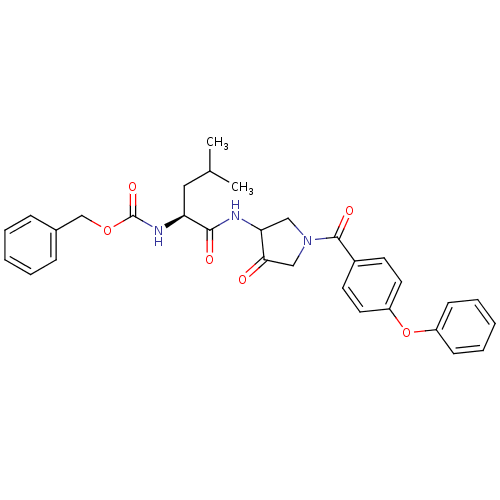
Affinity DataKi: 0.00480nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -52.1kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 0.730nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.06nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.42nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.44nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.58nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nM ΔG°: -49.7kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -49.3kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nM ΔG°: -48.8kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nM ΔG°: -48.5kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 4.06nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Inhibitory activity of the compound against Rat cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -45.0kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 12.4nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Displacement of [3H]CGS21680 form human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibition of cathepsin LMore data for this Ligand-Target Pair

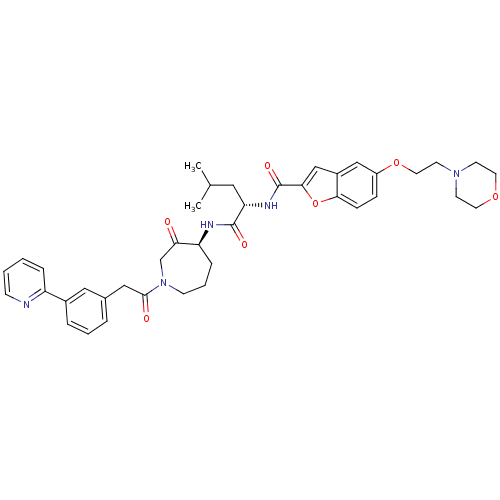
 3D Structure (crystal)
3D Structure (crystal)