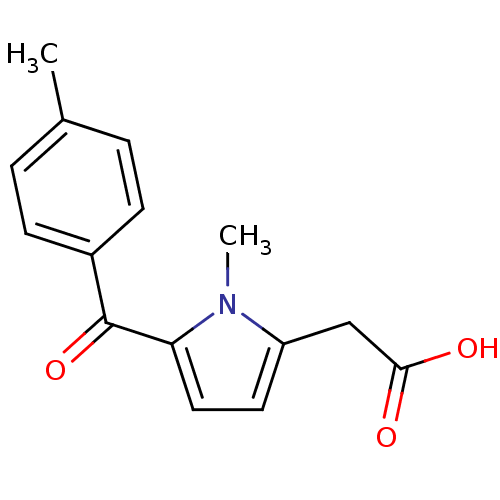
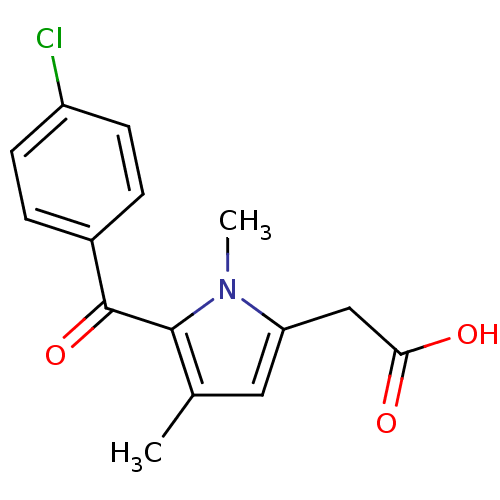
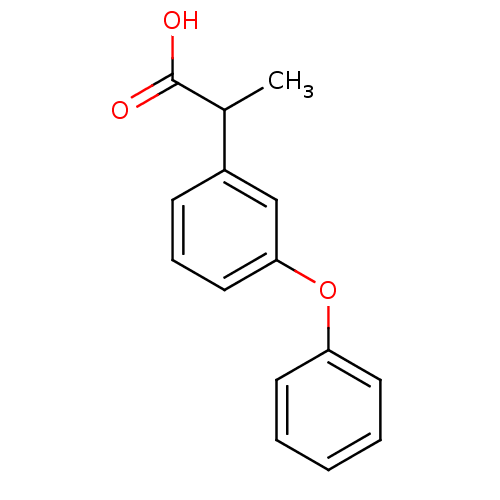
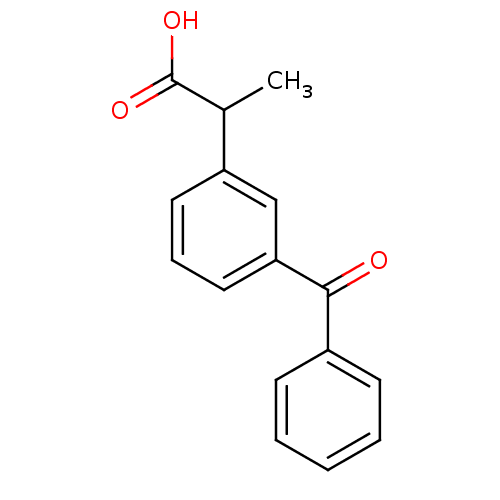
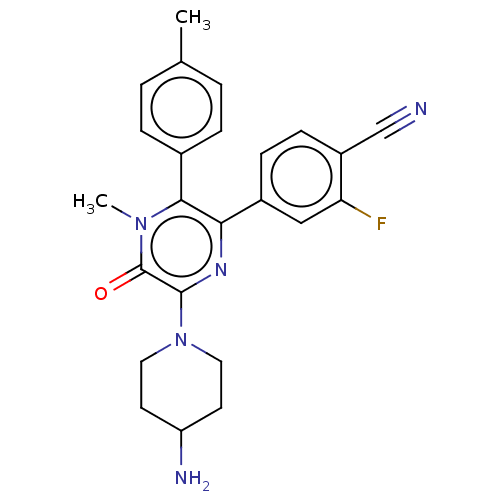
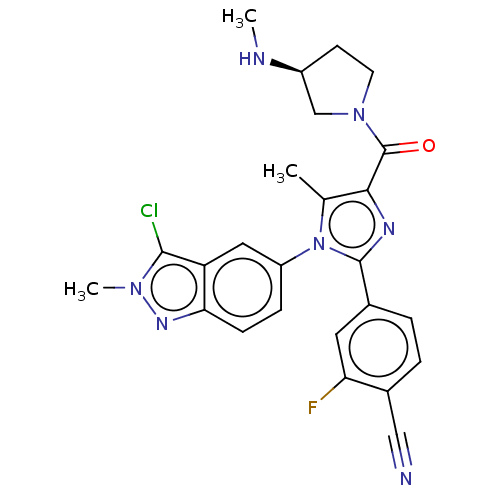
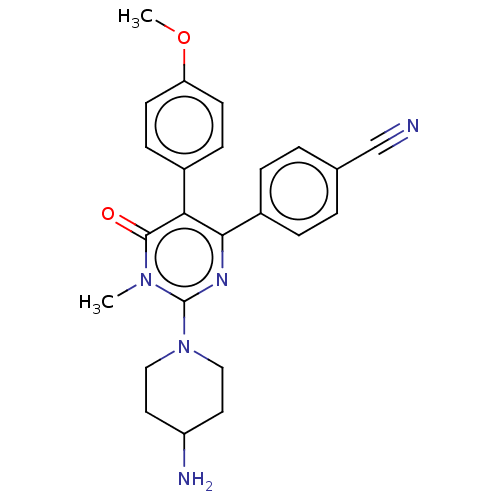
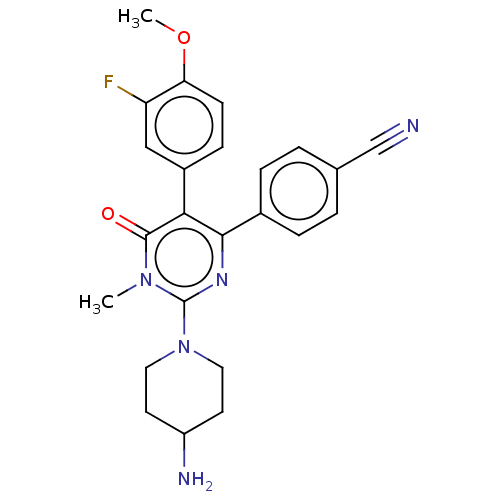
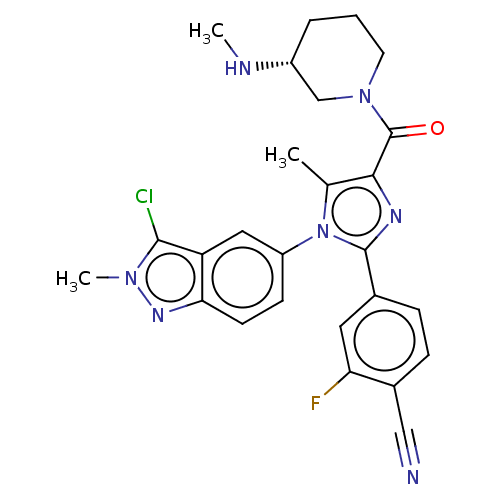
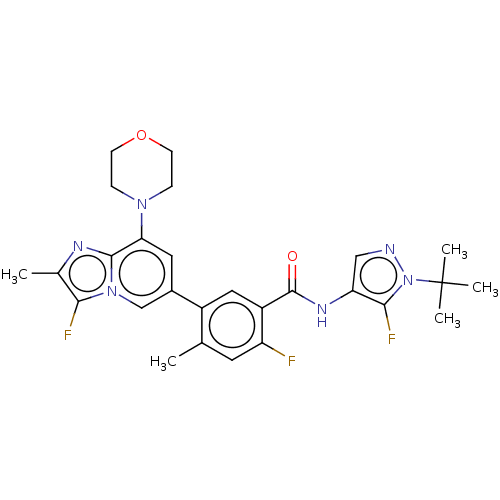
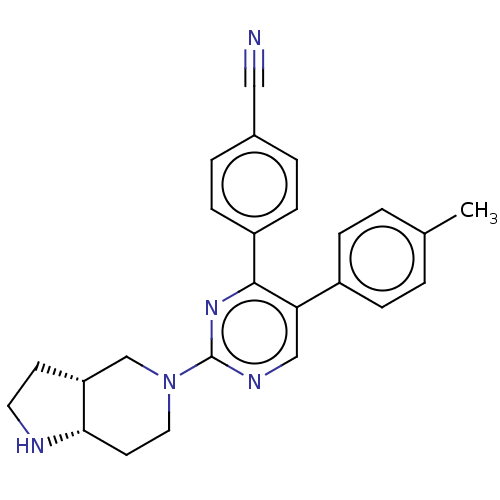
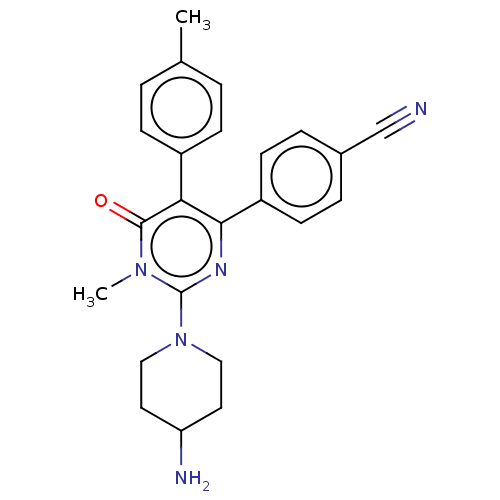
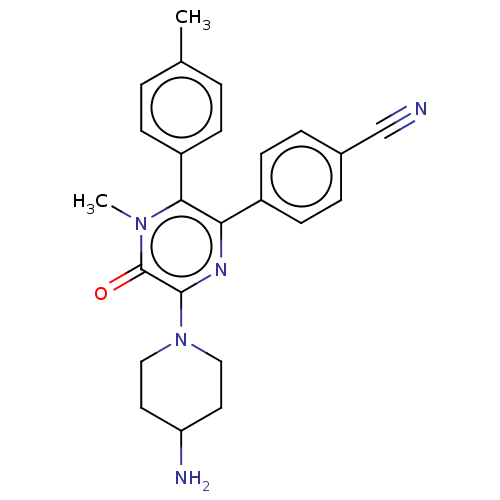
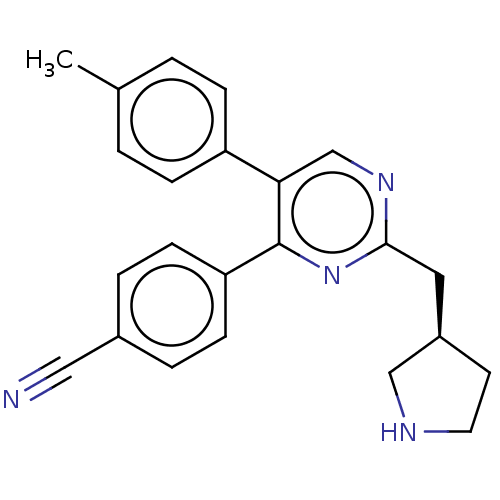
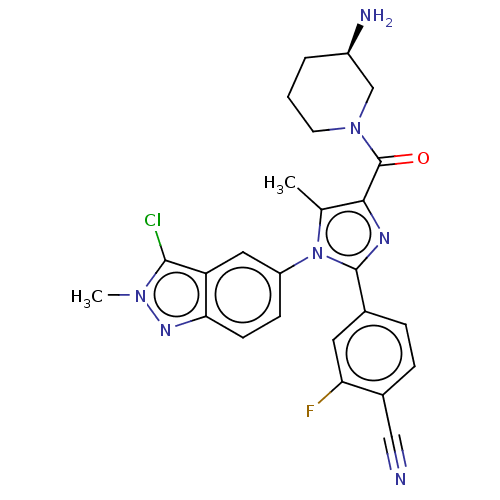
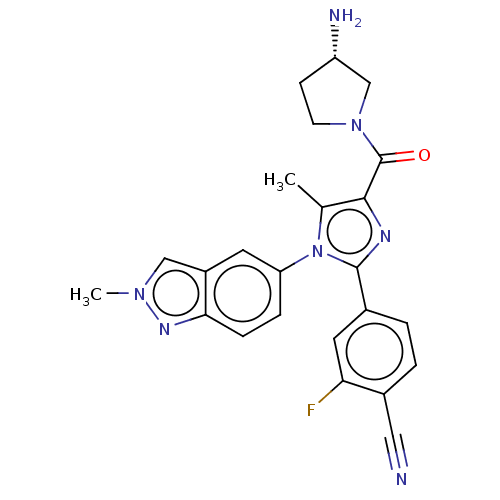
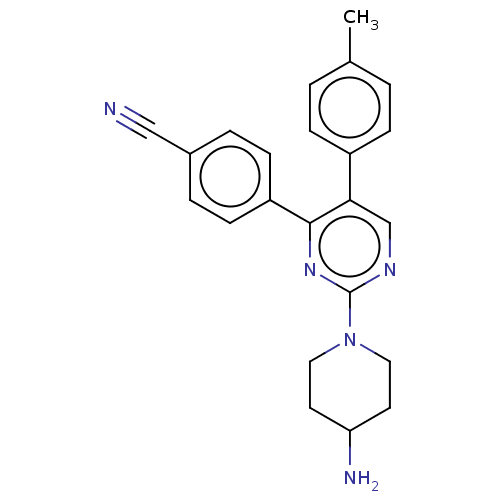
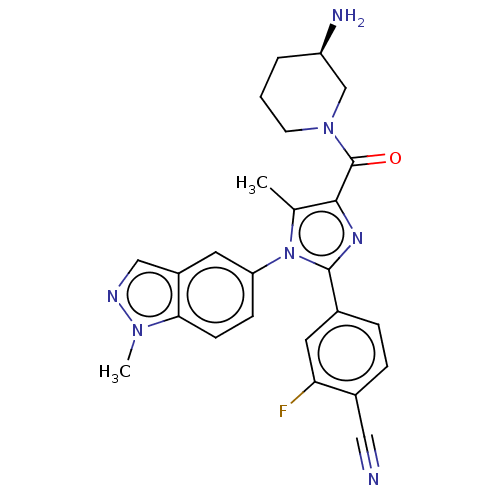
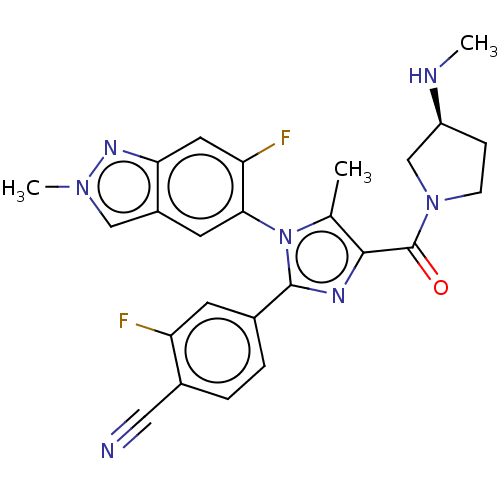
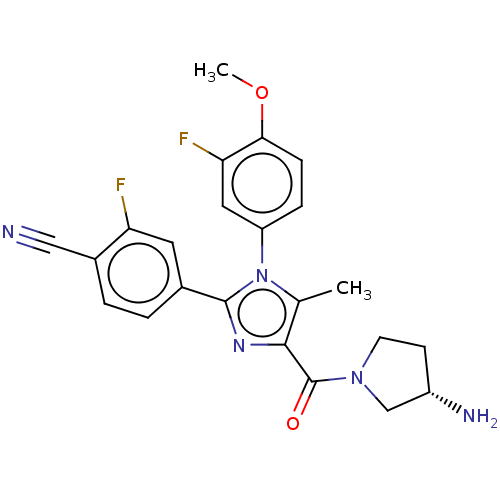
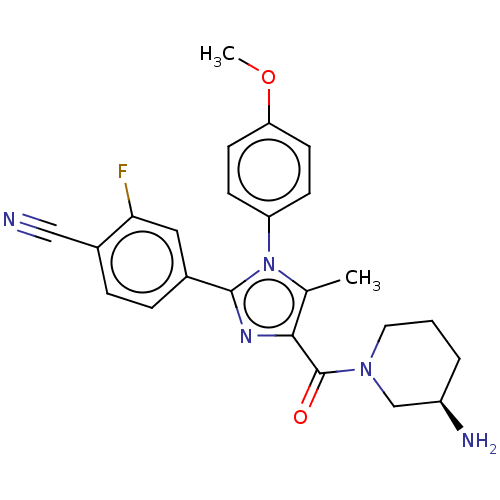
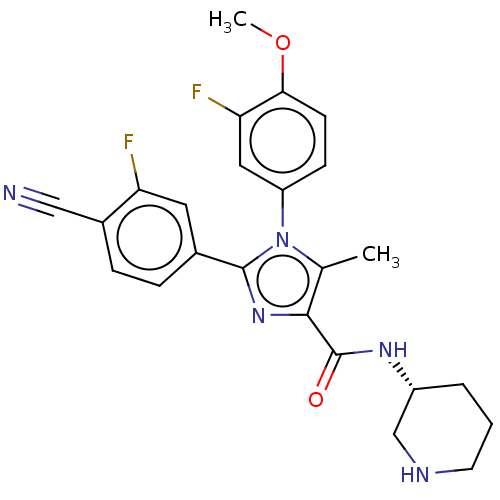
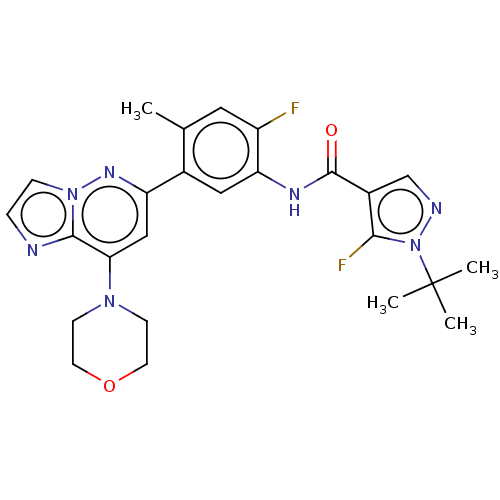
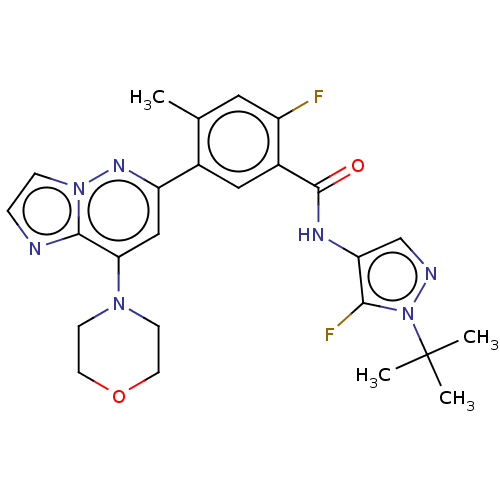
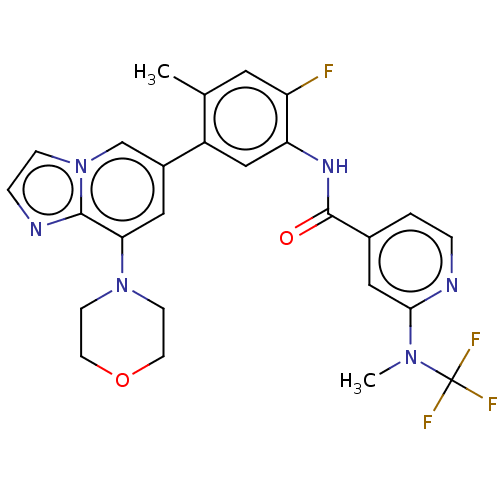
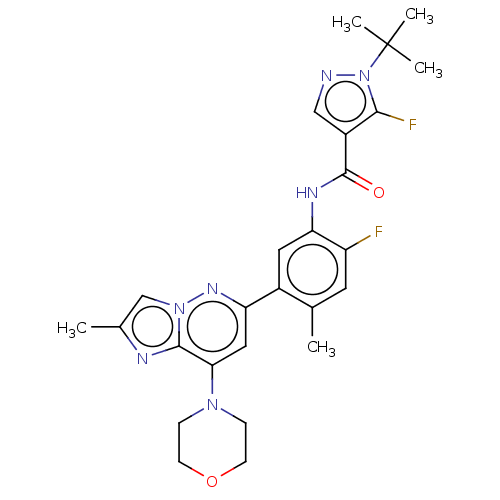
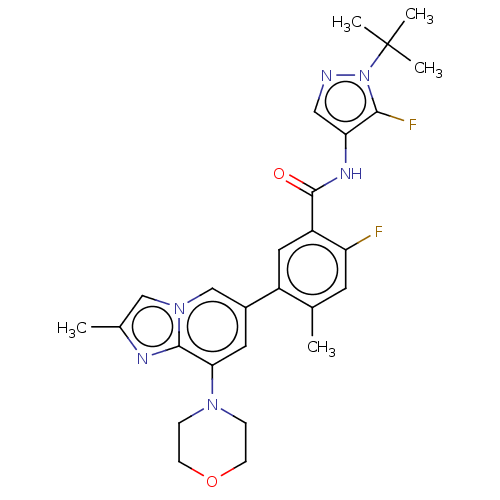
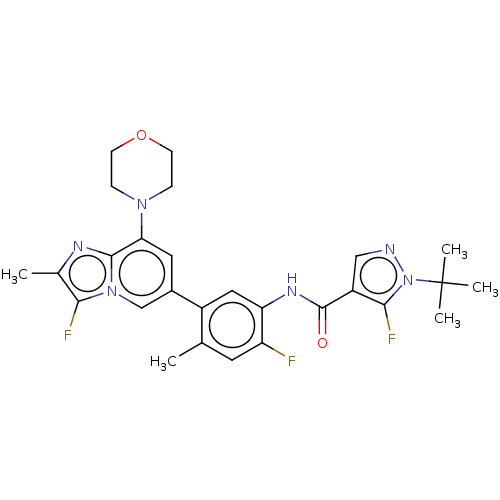
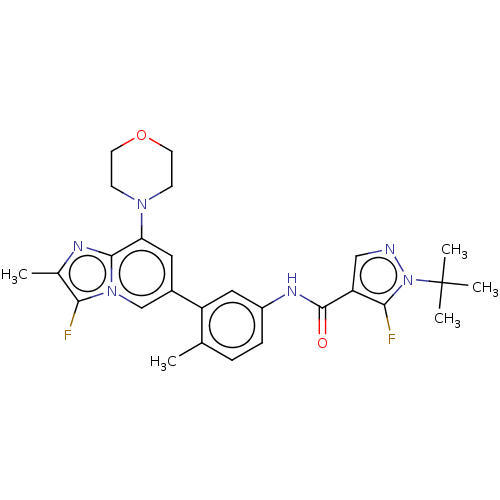
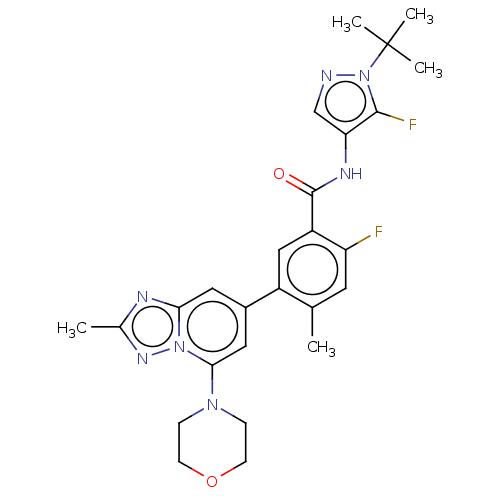
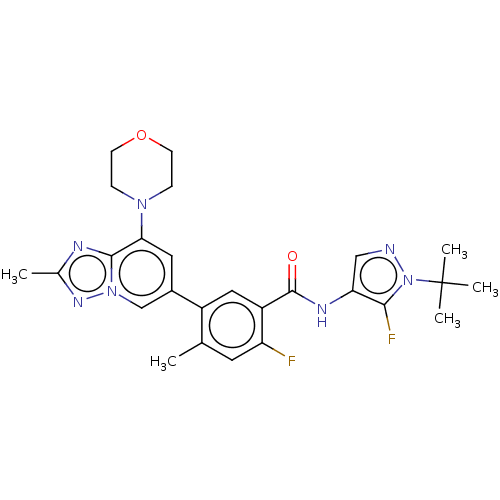
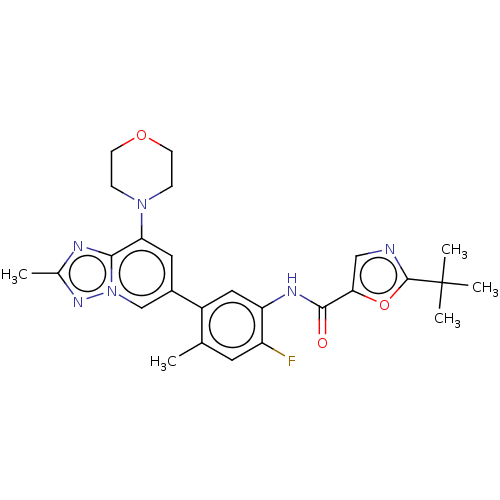
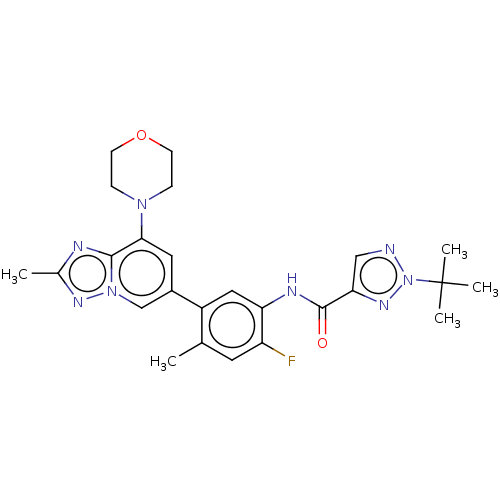
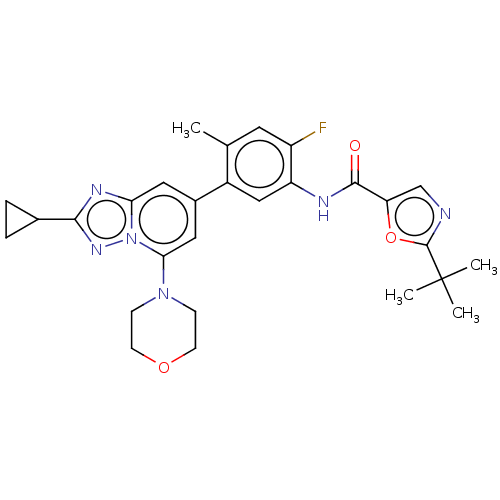
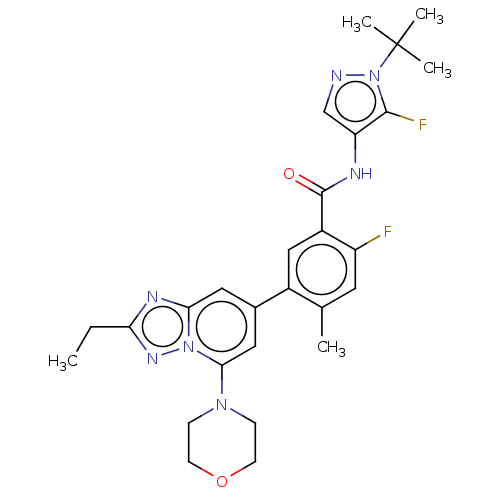
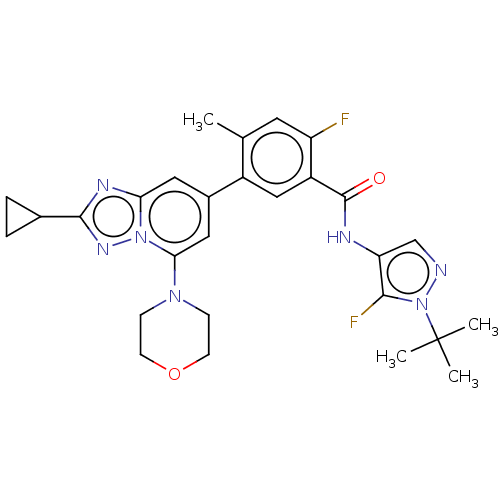
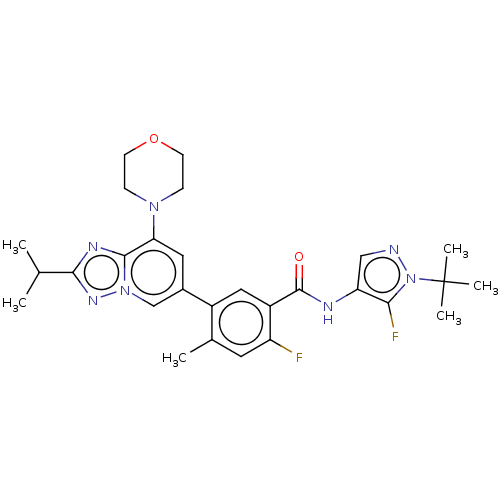
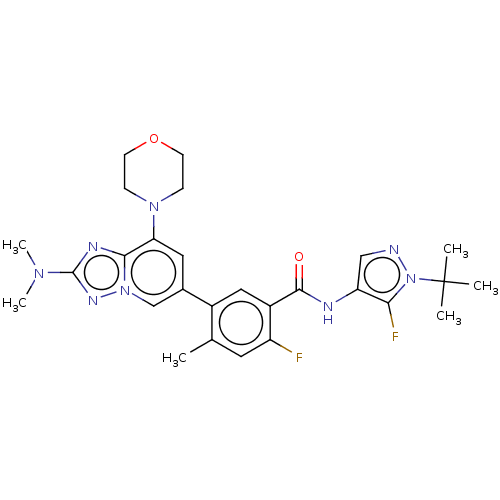
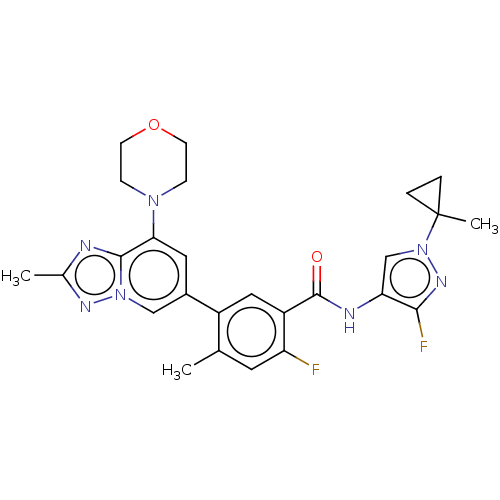
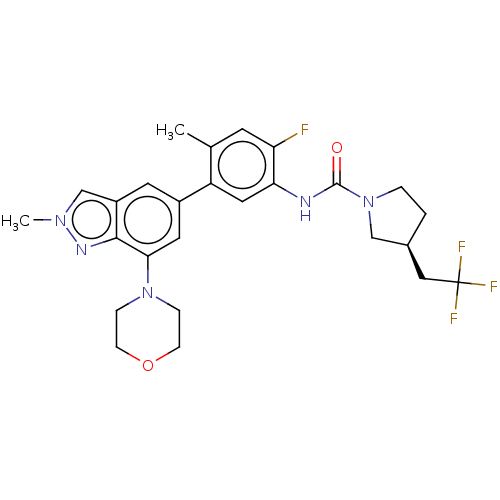
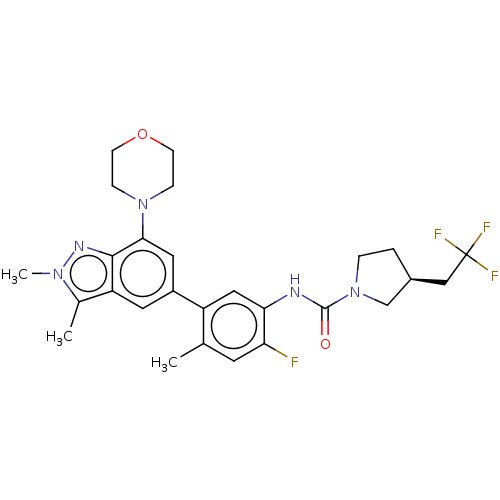
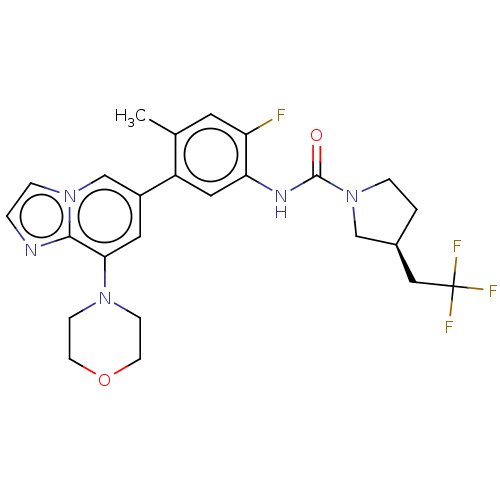
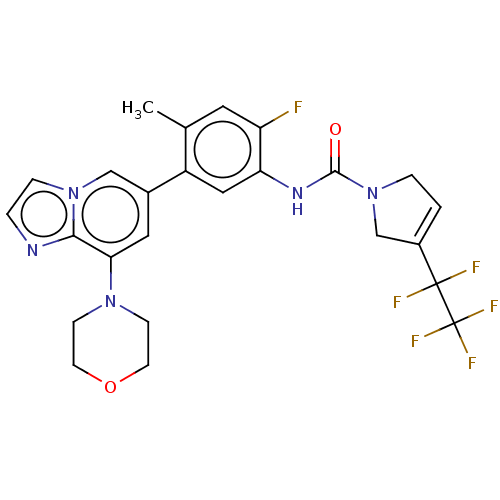
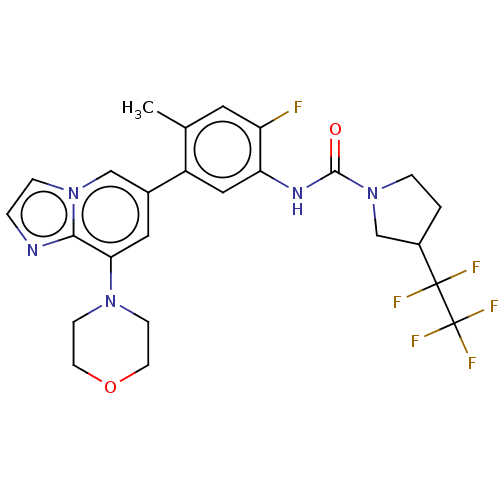
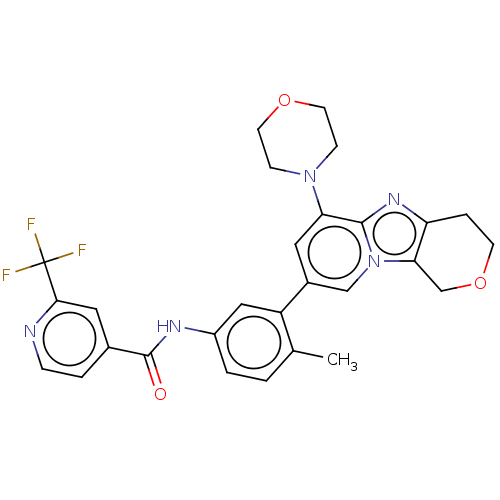
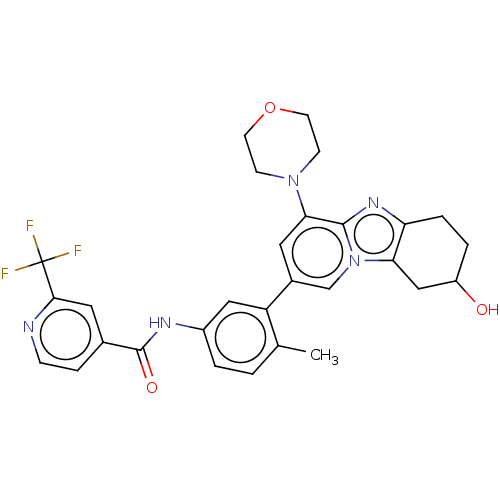
Affinity DataKi: 1.03E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 1.82E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 2.44E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 8.78E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 3.35E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 3.83E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 8.43E+5nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of LSD1 (unknown origin) using H3K4me1 substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Fount Therapeutics
Curated by ChEMBL
Fount Therapeutics
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Small molecule inhibition of the BRAF kinases was measured using ADP-Glo assay. In the assay, ADP is converted to ATP in the presence of test kinase ...More data for this Ligand-Target Pair

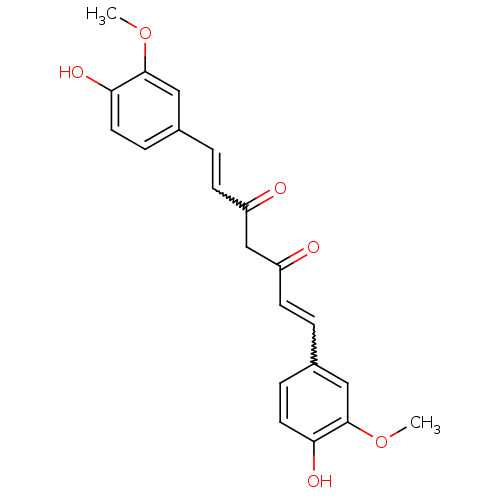
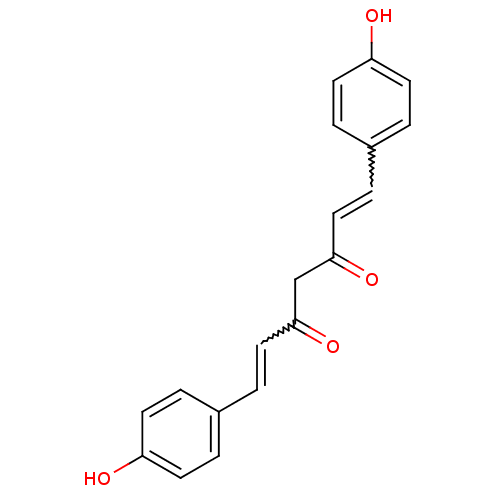

 3D Structure (crystal)
3D Structure (crystal)