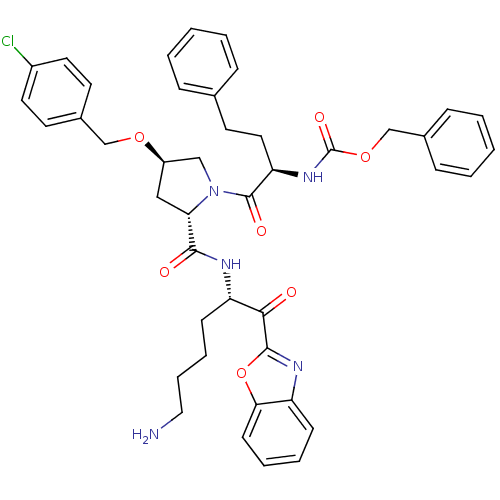
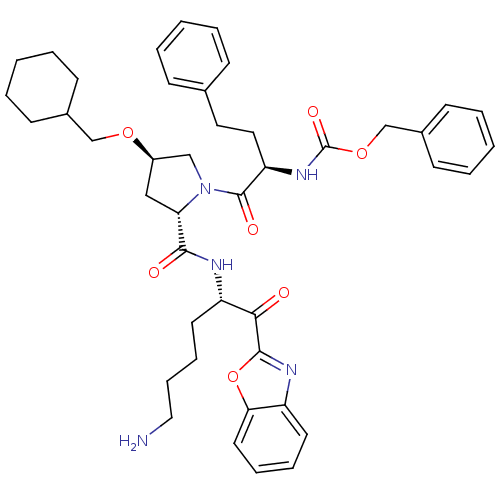
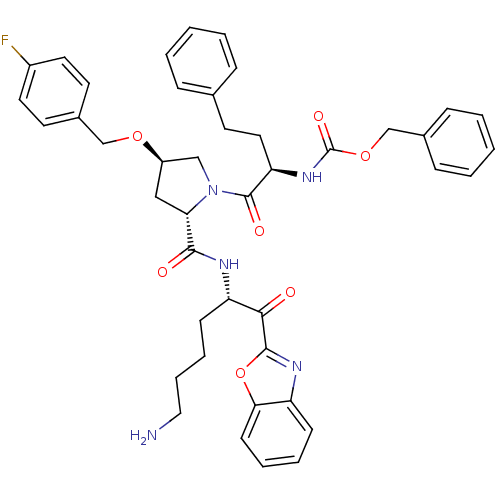
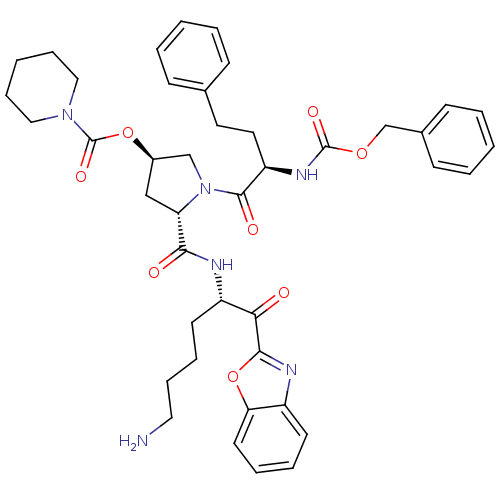
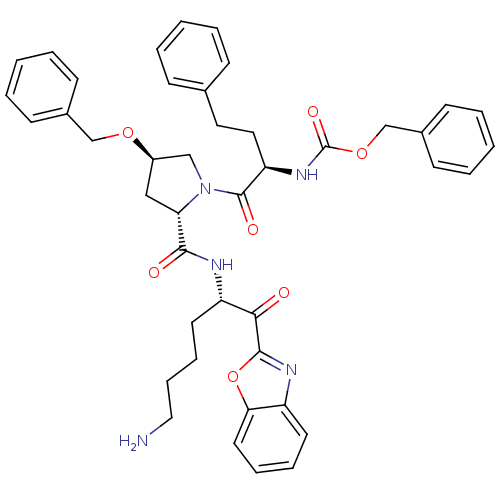
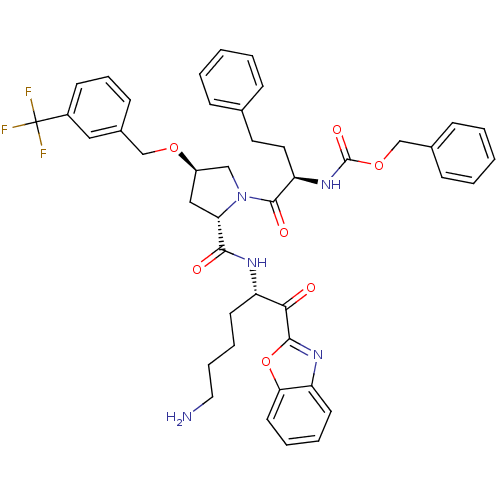
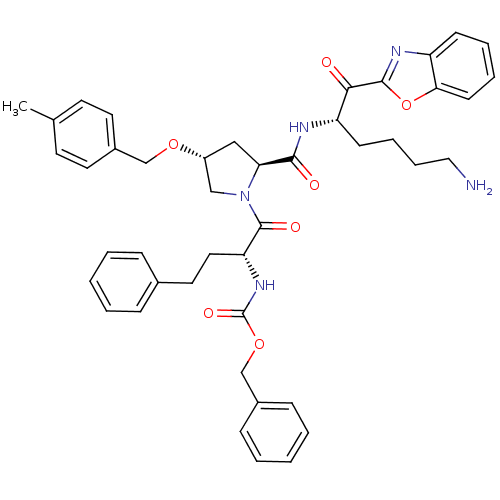
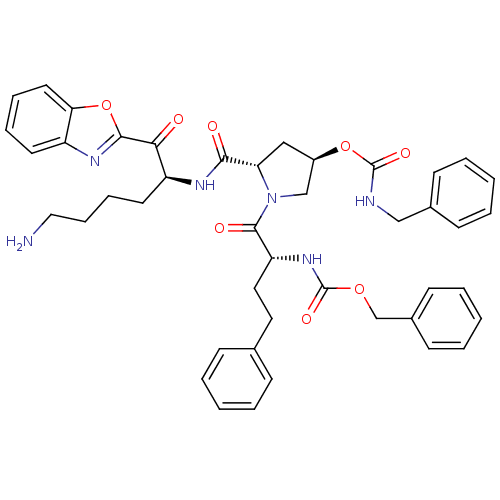
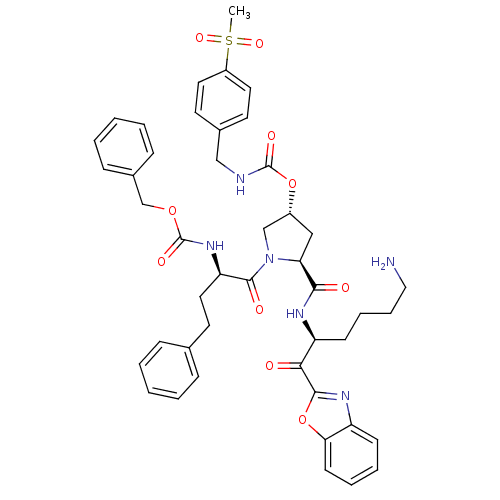
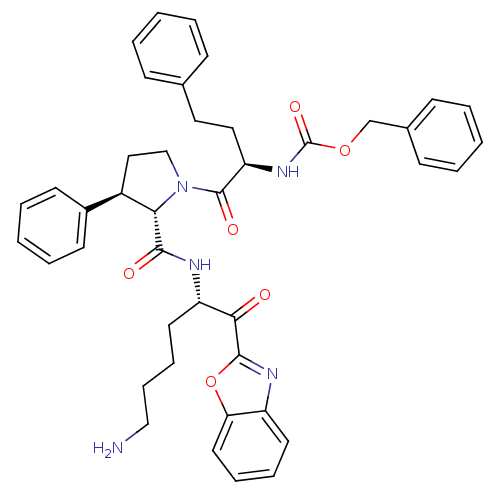
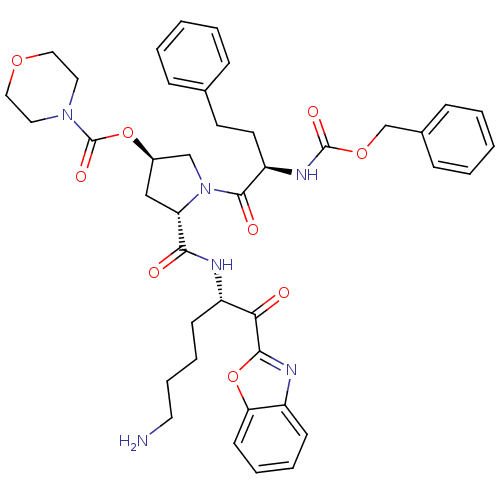
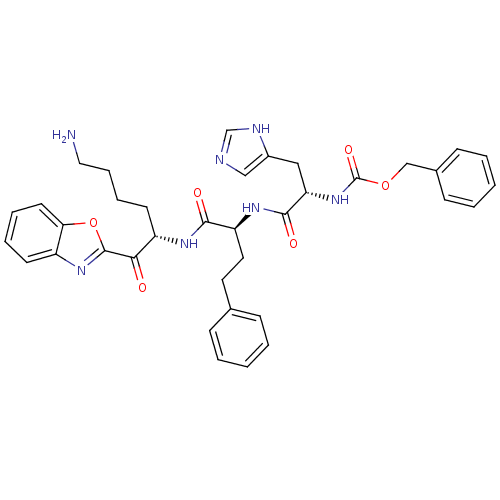
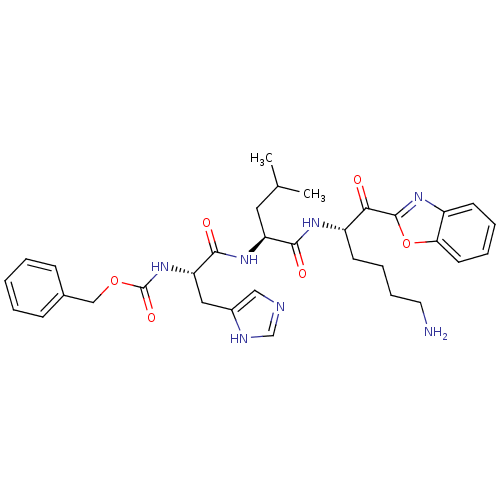
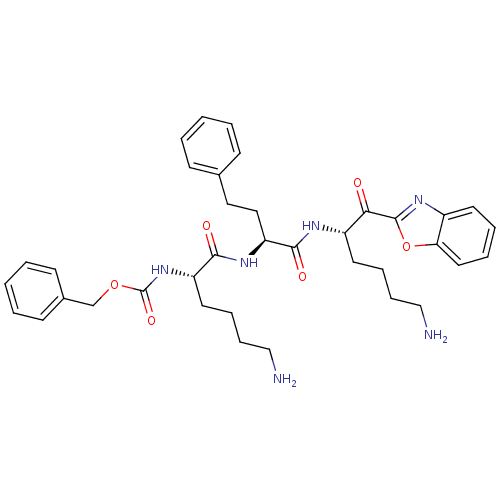
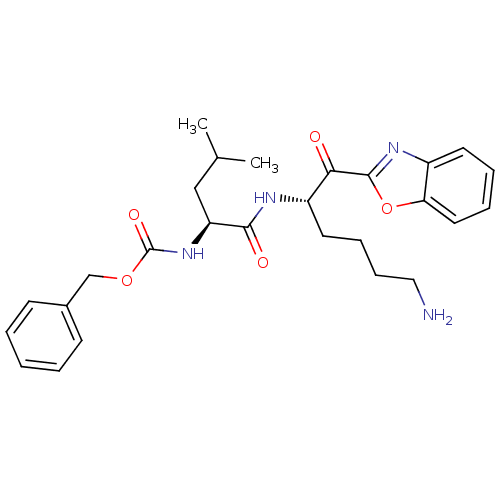
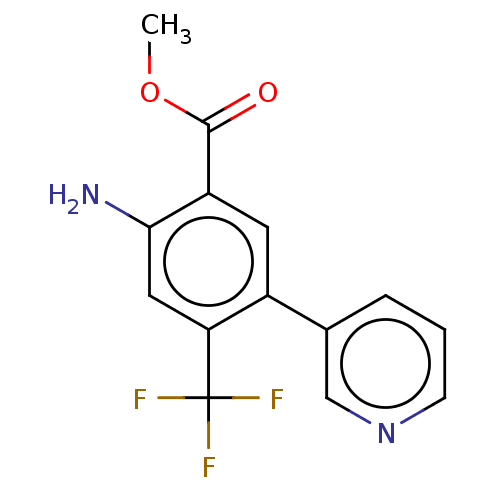
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 41nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 45nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 49nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 101nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 176nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 267nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 510nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.04E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.55E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 5.72E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 5.78E+3nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: 1.52E+4nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetProstasin(Homo sapiens (Human))
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Genomics Institute Of The Novartis Research Foundation
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to human prostasinMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Binding affinity to human ERG channelMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.08E+4nMAssay Description:Inhibition of human ERG channel by patch clamp assayMore data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 8.20E+3nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential preincubat...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 390nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential preincubat...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 920nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential preincubat...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 8.48E+3nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 1.25E+3nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 78nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 3.78E+3nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 3.00E+4nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 362nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 240nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 329nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 462nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 5.31E+3nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 5.83E+3nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 1.86E+3nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 3.00E+4nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 250nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 40nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 70nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 23nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 9nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 15nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 30nMAssay Description:Potentiation of CFTR F508del mutant (unknown origin) expressed in CHO cells assessed as chloride transport by measuring membrane potential incubated ...More data for this Ligand-Target Pair