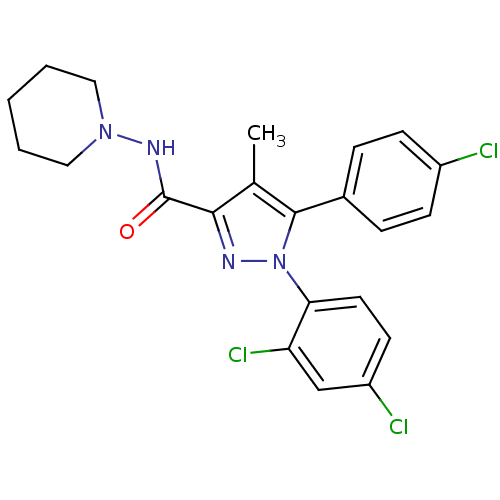
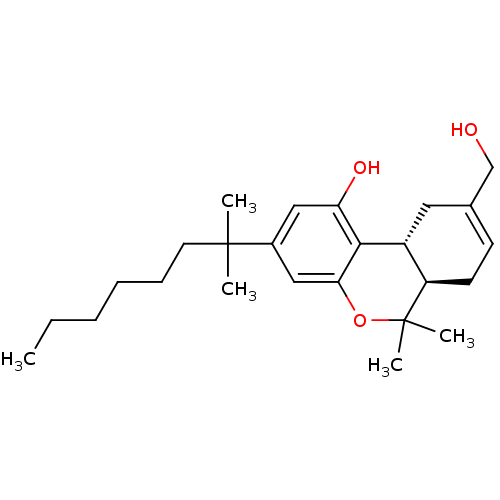
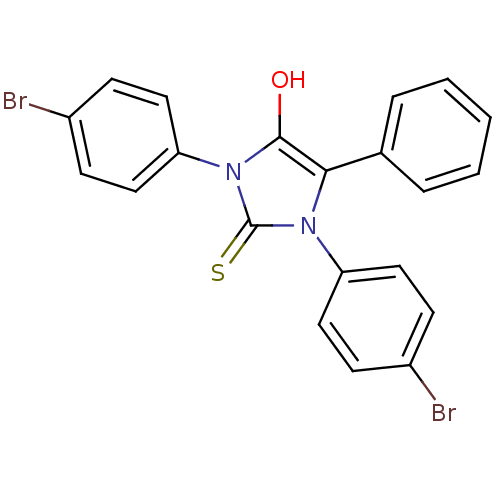
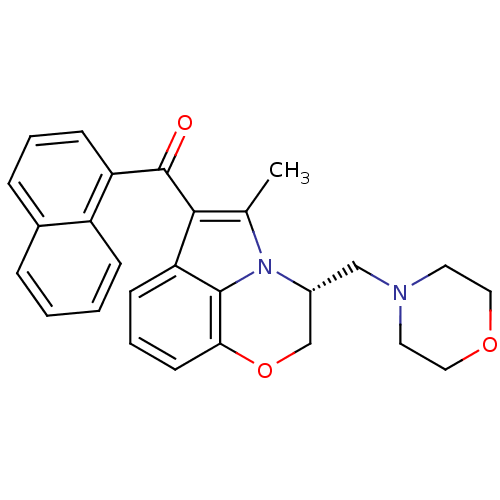
Affinity DataKi: 1.23nMAssay Description:Displacement of [3H]SR141716A from CB1 receptor in rat cerebellumMore data for this Ligand-Target Pair
Affinity DataKi: 2.75nMAssay Description:Displacement of [3H]SR141716A from CB1 receptor in rat cerebellumMore data for this Ligand-Target Pair
Affinity DataKi: 5.40nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 18.6nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 243nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 247nMAssay Description:Displacement of [3H]SR141716A from CB1 receptor in rat cerebellumMore data for this Ligand-Target Pair
Affinity DataKi: 311nMAssay Description:Displacement of [3H]SR141716A from CB1 receptor in rat cerebellumMore data for this Ligand-Target Pair
Affinity DataKi: 353nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 692nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 905nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.45E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.74E+3nMAssay Description:Displacement of [3H]SR141716A from CB1 receptor in rat cerebellumMore data for this Ligand-Target Pair
Affinity DataKi: 1.88E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.11E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.16E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.62E+3nMAssay Description:Displacement of [3H]SR141716A from CB1 receptor in rat cerebellumMore data for this Ligand-Target Pair
Affinity DataKi: 3.31E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.03E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.97E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.31E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.46E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.66E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.03E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >8.00E+3nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 10.1nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.10E+3nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 309nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 936nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 461nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.62E+3nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 195nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.23E+3nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.600nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.12E+3nMAssay Description:Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assayMore data for this Ligand-Target Pair