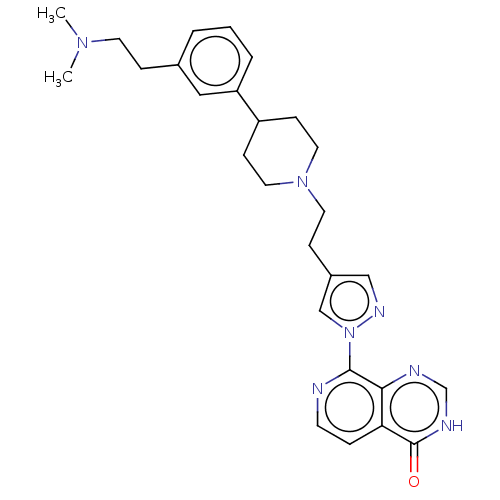
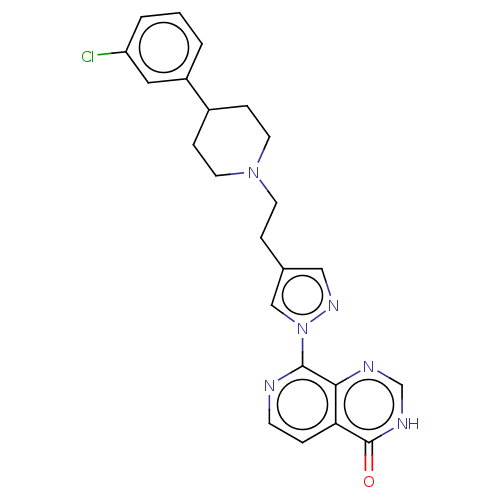
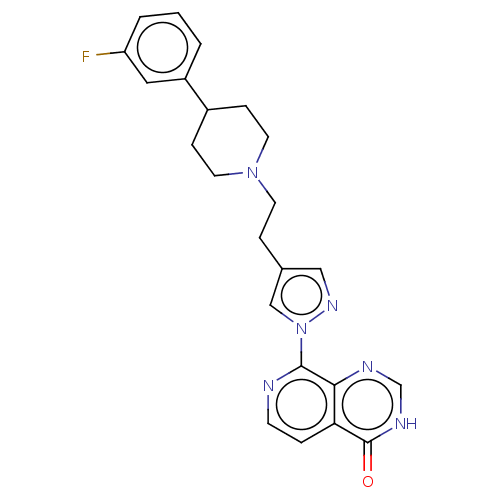
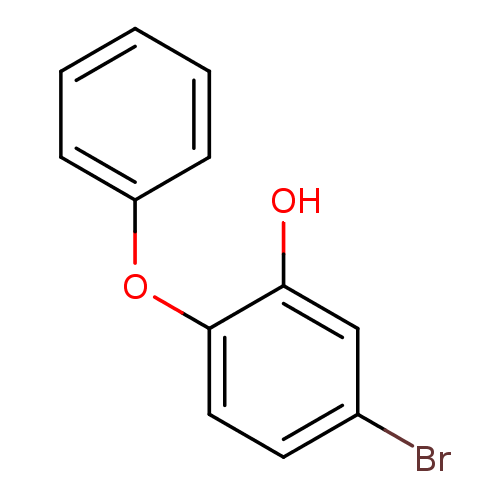
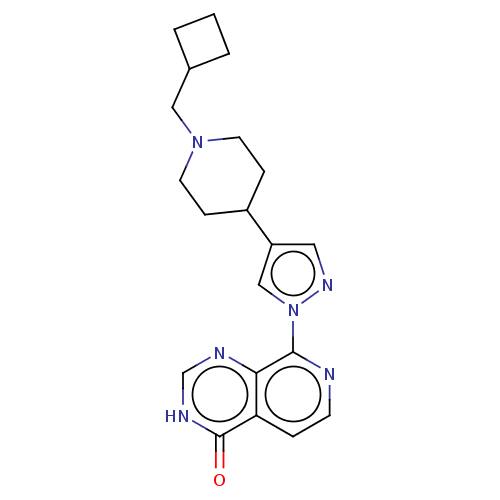
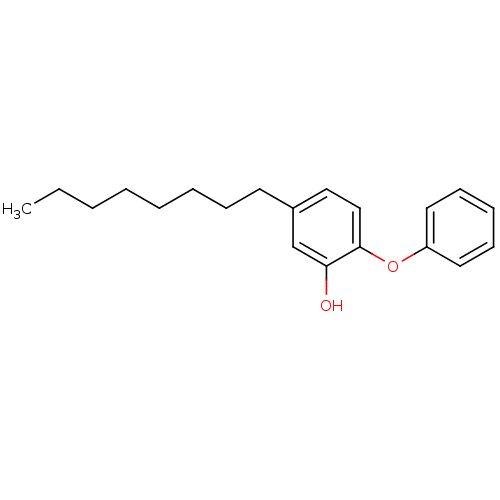
Affinity DataKi: 0.0510nM ΔG°: -58.7kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
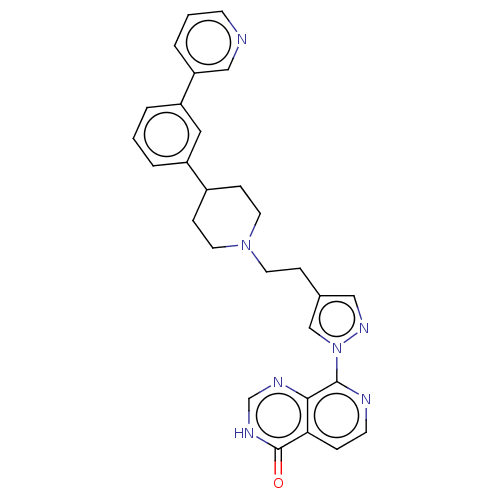
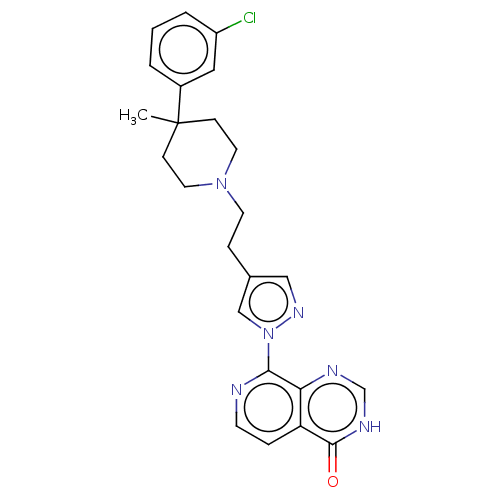
Affinity DataKi: 0.440nM ΔG°: -53.4kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.30nM ΔG°: -50.7kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -49.8kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -49.8kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.10nM ΔG°: -49.5kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
Affinity DataKi: 2.70nM ΔG°: -48.9kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 7.10nM ΔG°: -46.5kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Mus musculus (mouse))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
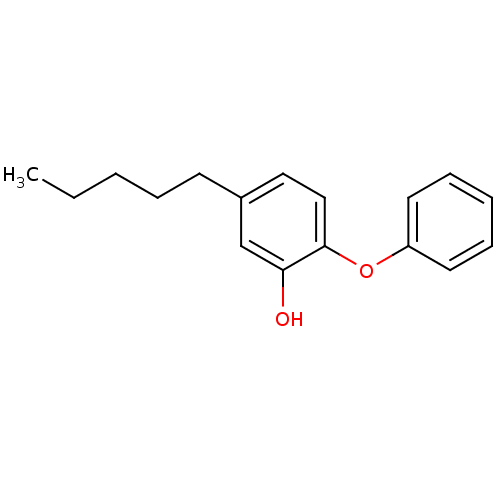
Affinity DataKi: 10nMAssay Description:Displacement of [3H]-prostaglandin D2 from mouse CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetLysine-specific demethylase 5B(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Binding affinity to KDM5B (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 23.6nM ΔG°: -43.5kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetLysine-specific demethylase 4A(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Binding affinity to KDM4A (unknown origin)More data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
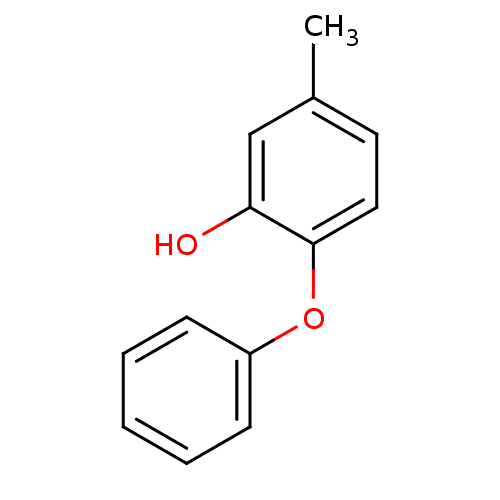
Affinity DataKi: 31nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of human recombinant KDM1A expressed in Escherichia coli using H3K4me as substrate by peroxidase coupled enzyme assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataKi: 59nMAssay Description:Inhibition of GST-tagged KDM1A (unknown origin) expressed in Escherichia coli BL21-CodonPlus-(DE3)-RIPL cells using dimethyl-Lys-4 H3-21 as substrate...More data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 103nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 109nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataKi: 140nMAssay Description:Inhibition of human recombinant His-tagged KDM1A (171 to 836 residues) using H3K4me2 as substrate by peroxidase coupled enzyme assayMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 145nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 147nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
Affinity DataKi: 149nM ΔG°: -39.0kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 149nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
Affinity DataKi: 192nM ΔG°: -38.3kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 208nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataKi: 210nMAssay Description:Inhibition of human recombinant His-tagged KDM1A (171 to 836 residues) using H3K4me2 as substrate by peroxidase coupled enzyme assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataKi: 240nMAssay Description:Inhibition of human recombinant His-tagged KDM1A (171 to 836 residues) using H3K4me2 as substrate by peroxidase coupled enzyme assayMore data for this Ligand-Target Pair
Affinity DataKi: 247nM ΔG°: -37.7kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 249nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 270nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 278nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
Affinity DataKi: 280nMAssay Description:Competitive inhibition of recombinant His6-tagged KDM2A (1 to 517 residues) (unknown origin) expressed in Escherichia coli BL21 cells using methylsta...More data for this Ligand-Target Pair
Affinity DataKi: 289nM ΔG°: -37.3kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NAD+More data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 302nMAssay Description:Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrsMore data for this Ligand-Target Pair
Affinity DataKi: 308nM ΔG°: -37.2kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair
Affinity DataKi: 311nM ΔG°: -37.1kJ/molepH: 8.0 T: 2°CAssay Description:Inhibition constant binding to E-NADHMore data for this Ligand-Target Pair

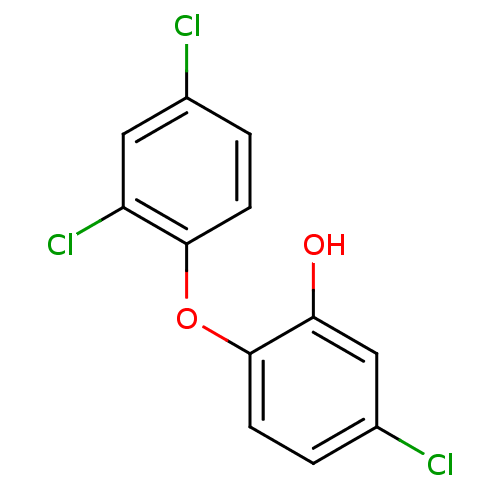
 3D Structure (crystal)
3D Structure (crystal)