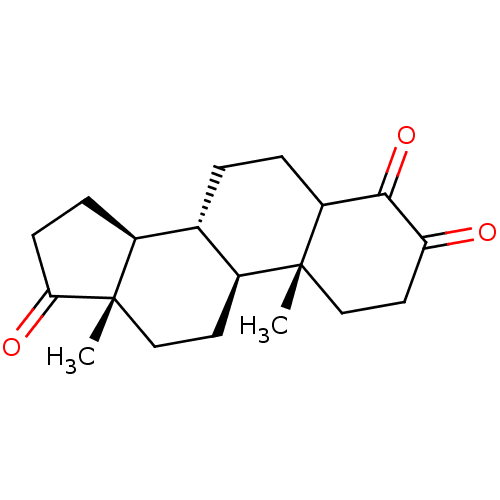
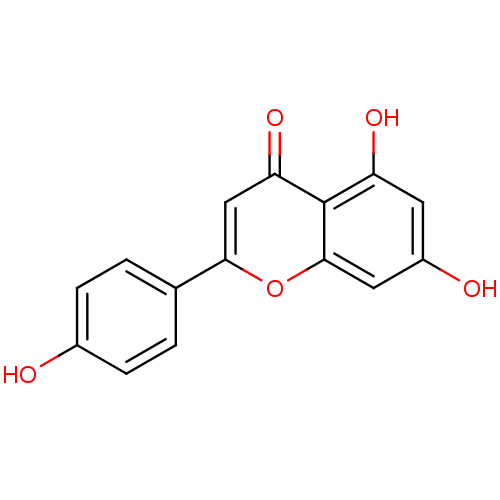
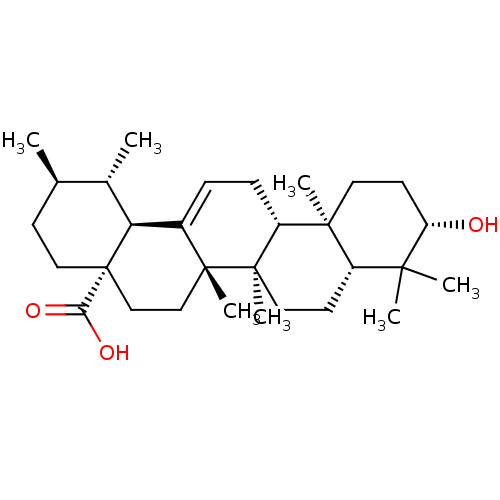
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Universidade Federal Do Rio Grande Do Sul (Ufrgs)
Curated by ChEMBL
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibition of aromatase in human placental microsomes assessed as tritiated water release after 15 mins using [1beta, 3H]androstenedione as substrate...More data for this Ligand-Target Pair