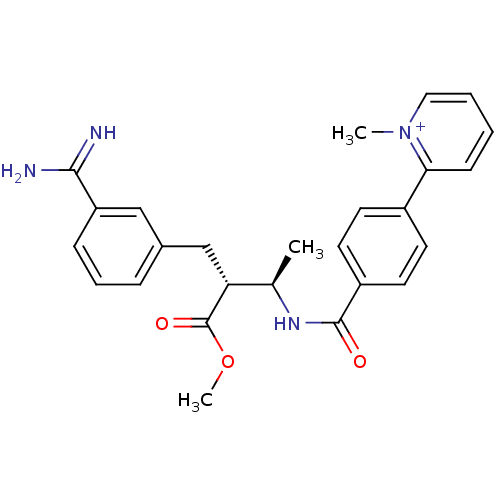
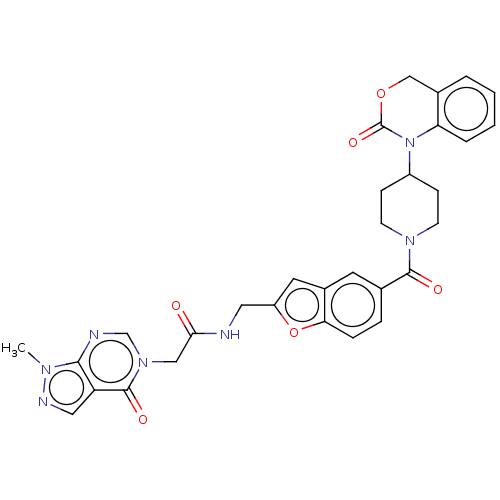
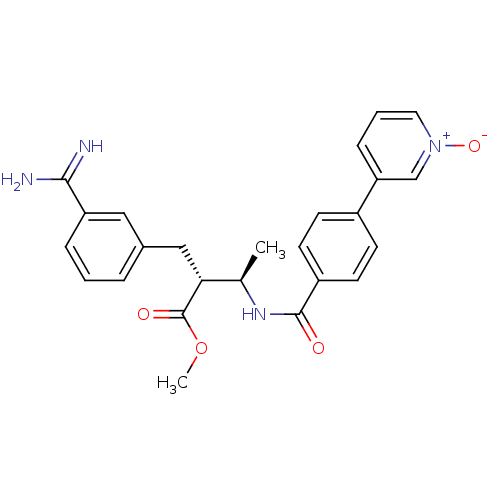
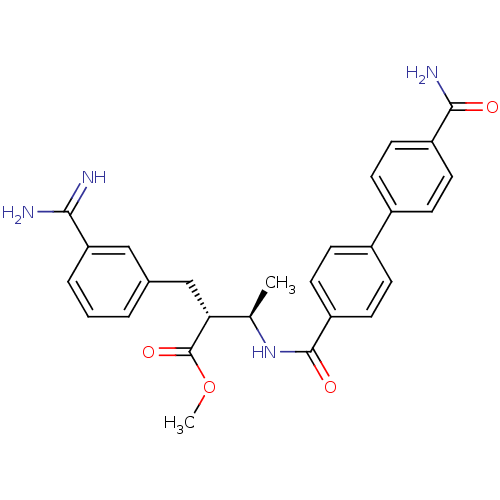
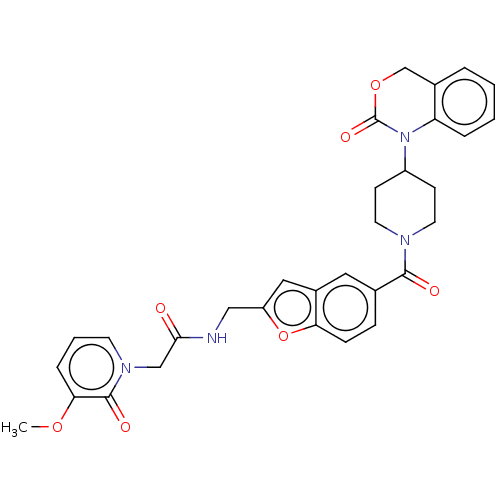
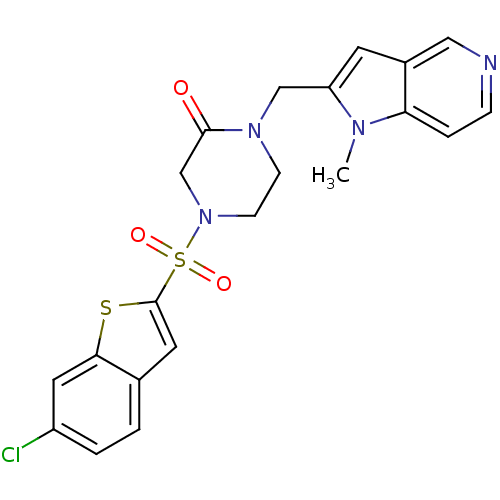
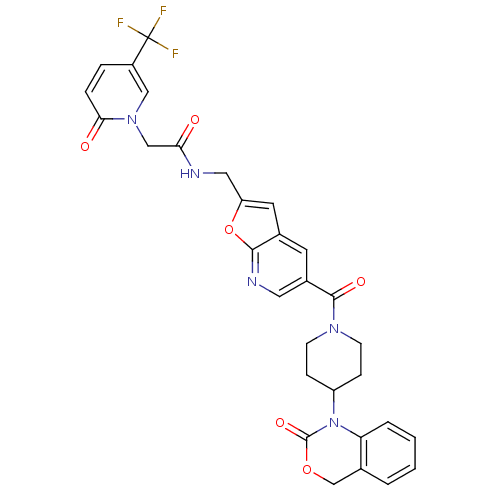
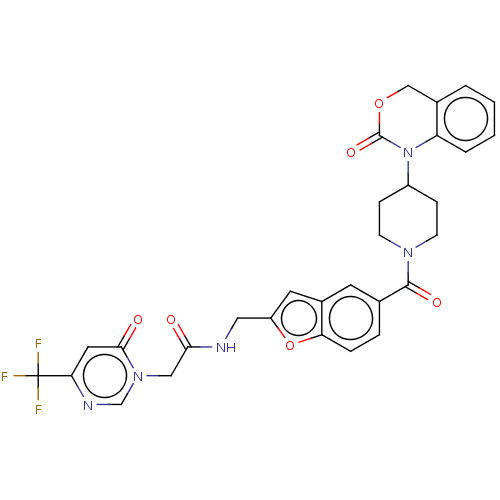
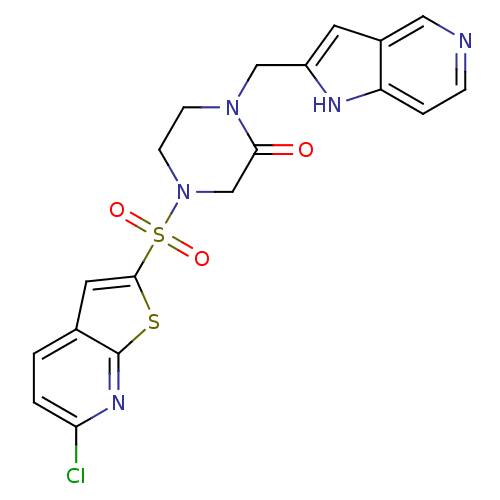
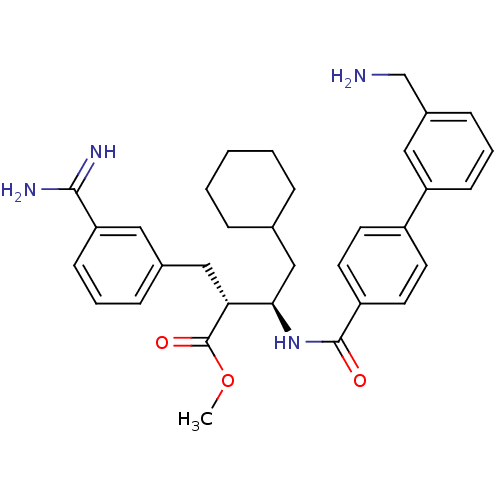
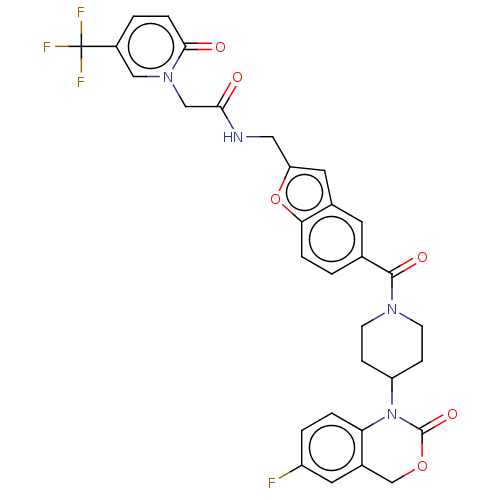
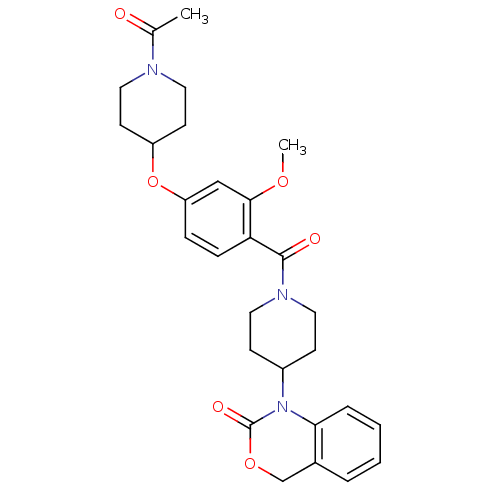
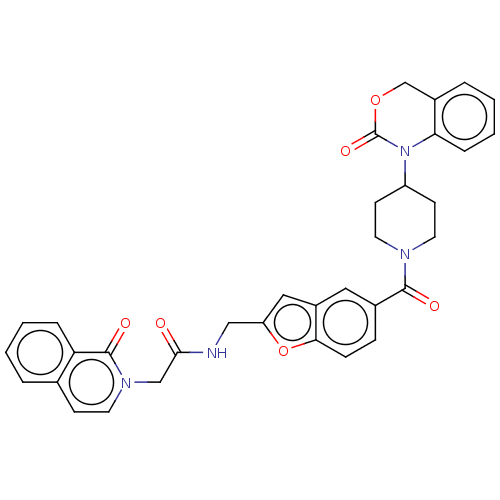
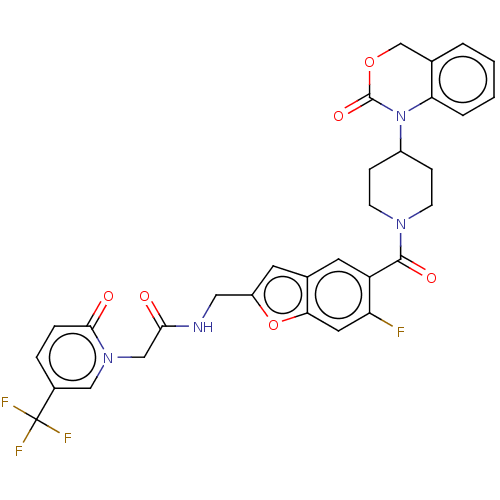
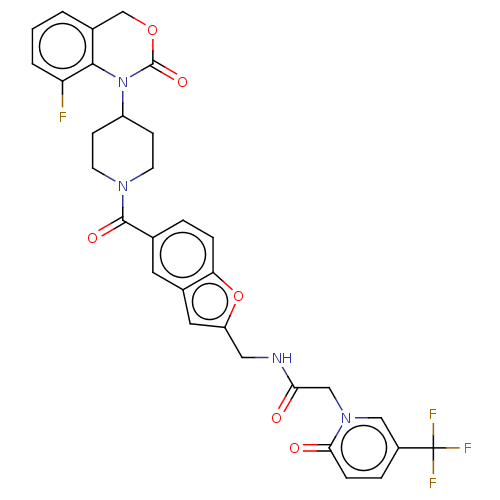
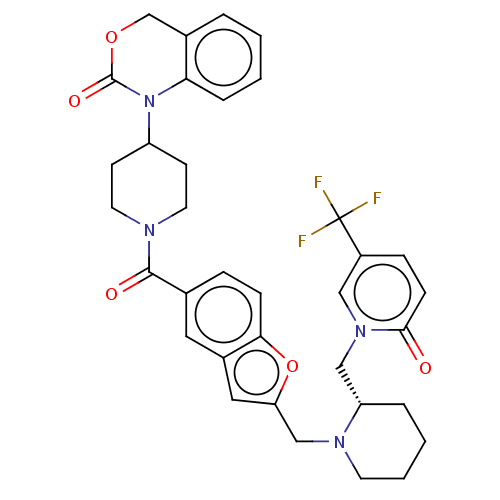
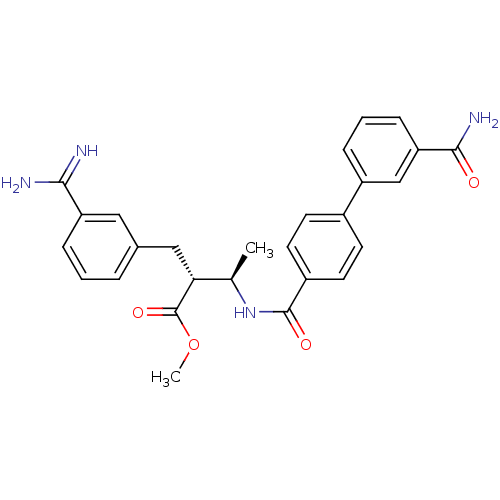
Affinity DataKi: 0.400nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
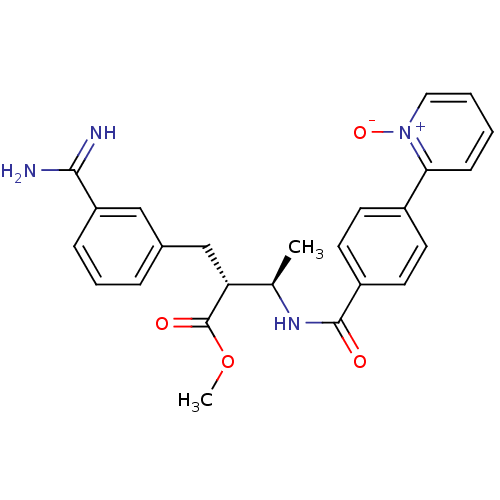
Affinity DataKi: 0.501nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.580nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.890nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 5 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 9.40nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:In vitro inhibitory activity against human Coagulation factor XaMore data for this Ligand-Target Pair








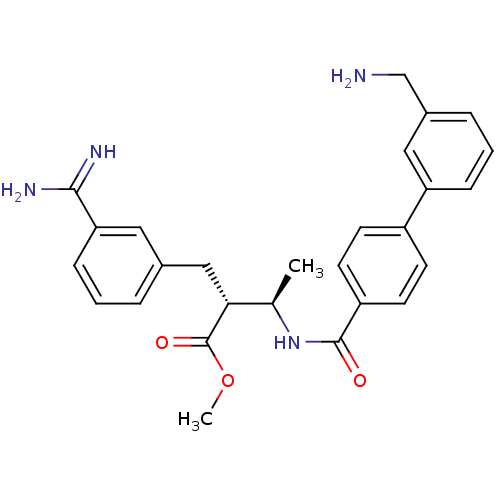
 3D Structure (crystal)
3D Structure (crystal)