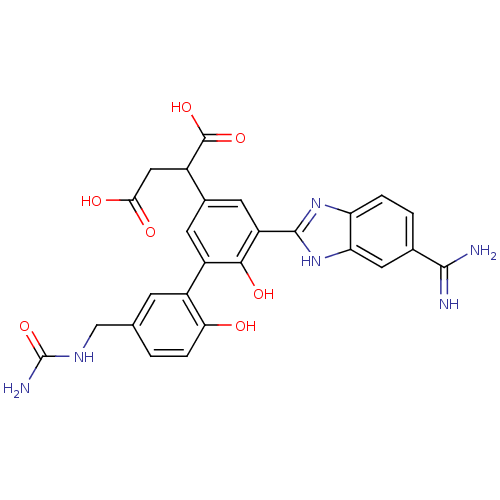
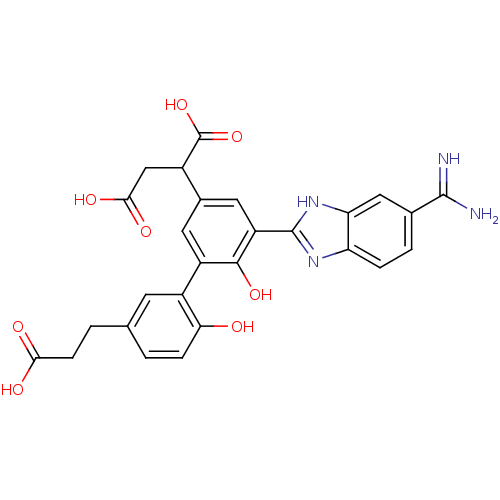
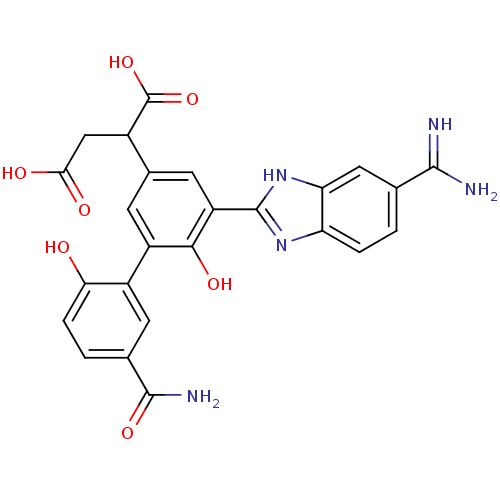
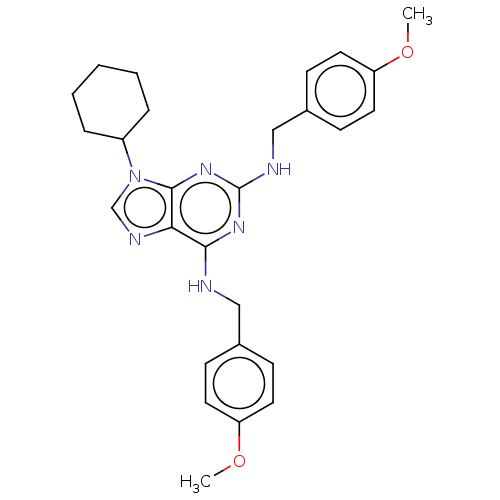
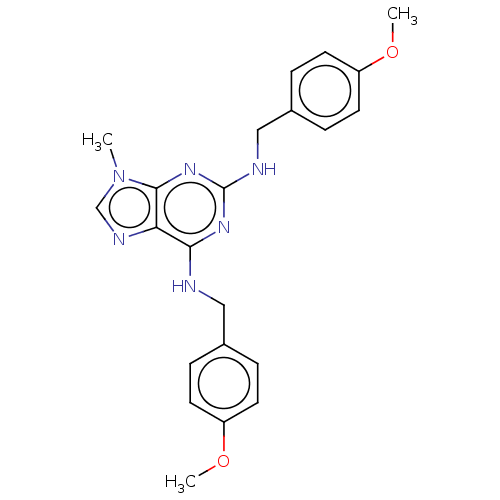
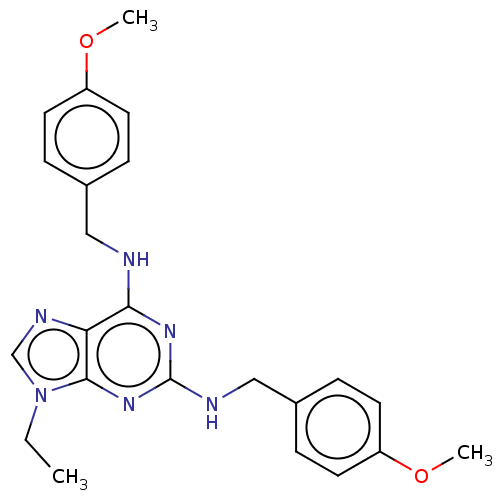
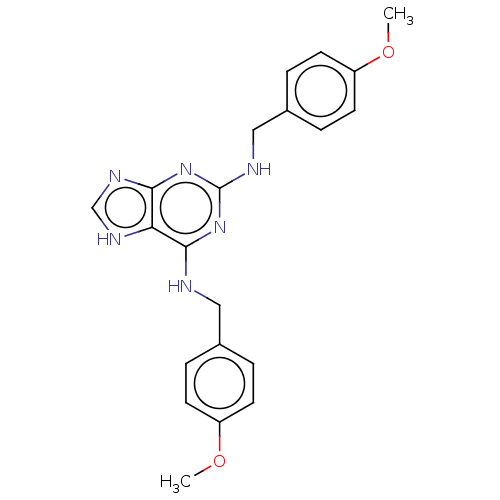
Affinity DataKi: 0.350nM ΔG°: -53.4kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 44nMAssay Description:Binding affinity to F7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 87nM ΔG°: -39.9kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 114nM ΔG°: -39.2kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nM ΔG°: -33.5kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >3.40E+3nM ΔG°: >-30.9kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >3.50E+3nM ΔG°: >-30.8kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nM ΔG°: -30.8kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >3.90E+3nM ΔG°: >-30.6kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nM ΔG°: -30.5kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 2.90E+4nMAssay Description:Inhibition of Pseudomonas aeruginosa aryl sulfatase after 20 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of tubulin polymerization using bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+5nMAssay Description:Inhibition of Klebsiella pneumoniae aryl sulfatase after 0.5 hrs by para-nitrocatechol sulfate-based colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+6nMAssay Description:Inhibition of Pseudomonas aeruginosa aryl sulfatase after 0.5 hrs by para-nitrocatechol sulfate-based colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+7nMAssay Description:Inhibition of Klebsiella pneumoniae aryl sulfatase after 20 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+7nMAssay Description:Displacement of 3H-DHEAS from human steroid sulfatase after 1 hr by liquid scintillation counting analysisMore data for this Ligand-Target Pair

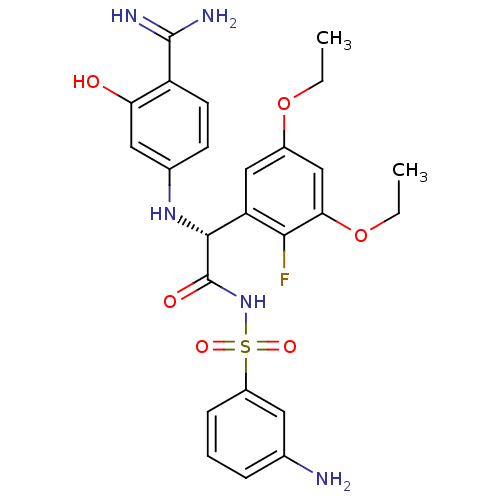
 3D Structure (crystal)
3D Structure (crystal)