Affinity DataKi: 1.54E+3nMAssay Description:Substrate inhibition of human UGT1A1-mediated T-5224 acyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC meth...More data for this Ligand-Target Pair
Affinity DataKi: 2.55E+3nMAssay Description:Substrate inhibition of human UGT1A3-mediated T-5224 hydroxyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC ...More data for this Ligand-Target Pair
Affinity DataKi: 3.64E+3nMAssay Description:Substrate inhibition of human UGT1A8-mediated T-5224 hydroxyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC ...More data for this Ligand-Target Pair
Affinity DataKi: 4.67E+3nMAssay Description:Substrate inhibition of human UGT1A1-mediated T-5224 hydroxyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC ...More data for this Ligand-Target Pair
Affinity DataKi: 1.35E+4nMAssay Description:Substrate inhibition of human UGT1A3-mediated T-5224 acyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC meth...More data for this Ligand-Target Pair
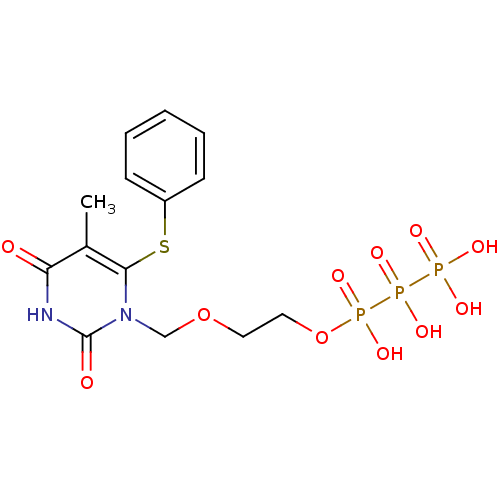
Affinity DataIC50: 34nMAssay Description:Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rA)-oligo(dT) template primerMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMAssay Description:Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rC)-oligo(dG) template primerMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+5nMAssay Description:Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rA)-oligo(dT) template primerMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+5nMAssay Description:Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rC)-oligo(dG) template primerMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+5nMAssay Description:Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rA)-oligo(dT) template primerMore data for this Ligand-Target Pair

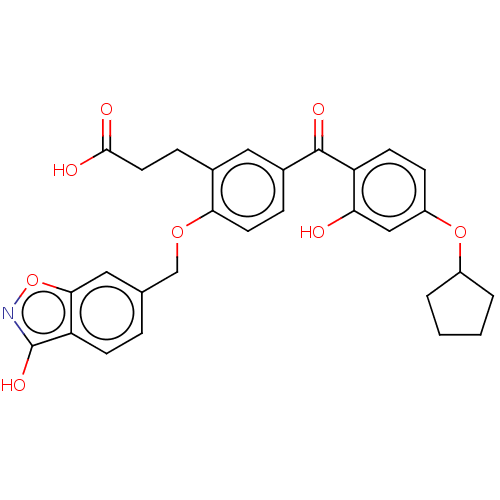


 3D Structure (crystal)
3D Structure (crystal)