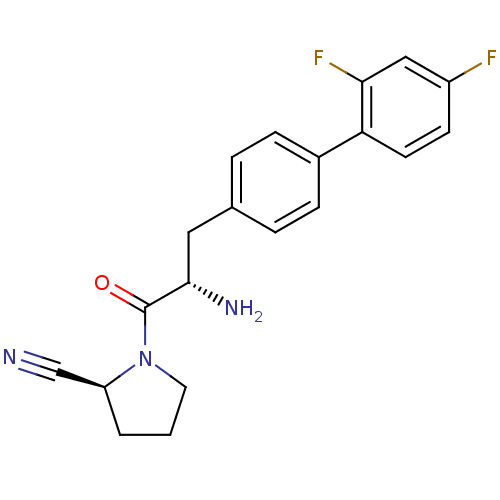
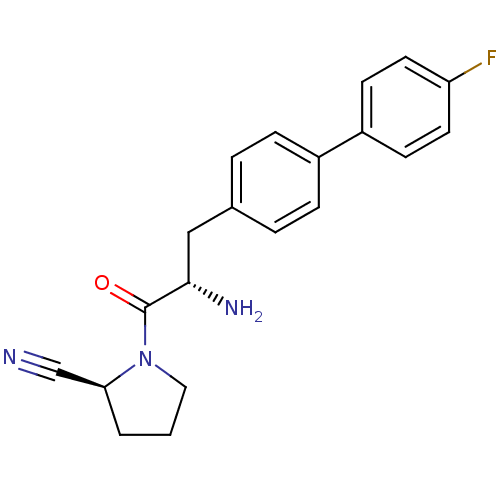
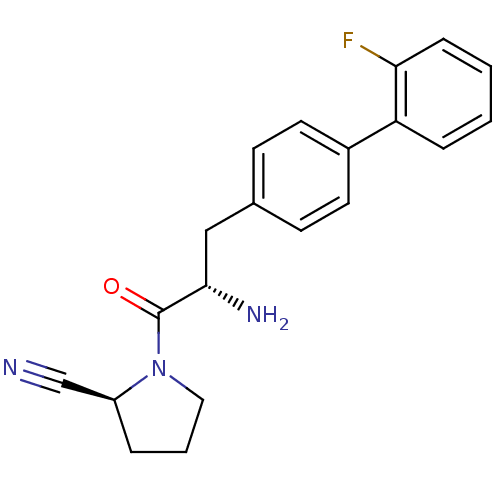
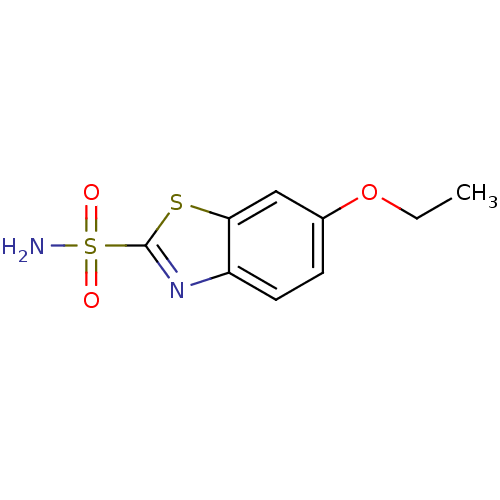
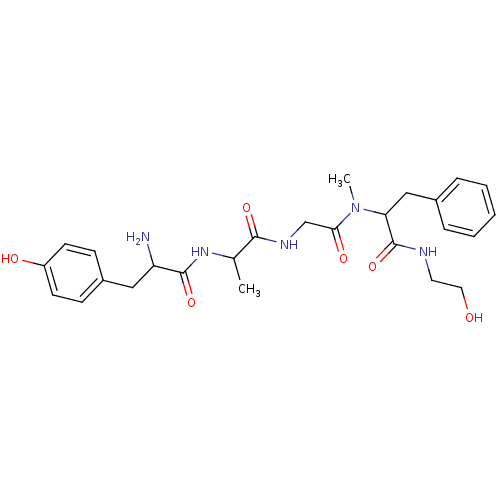
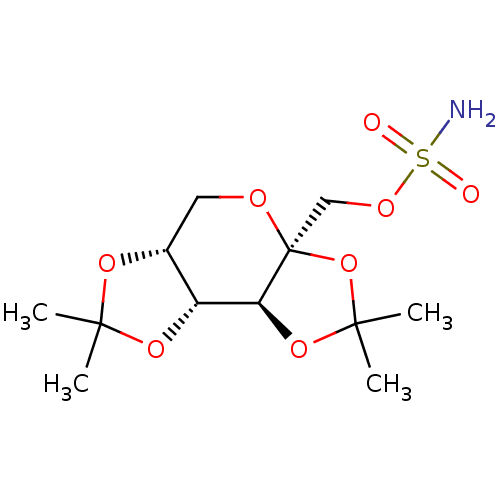
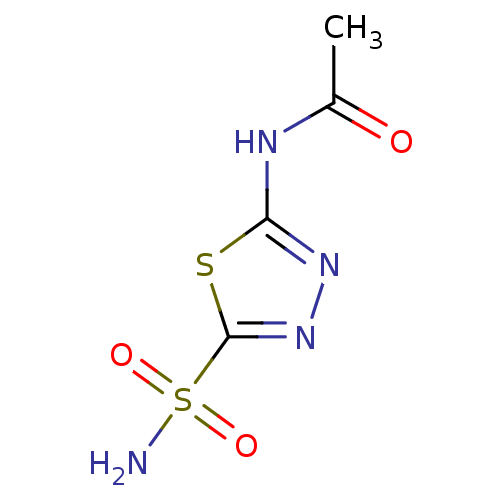
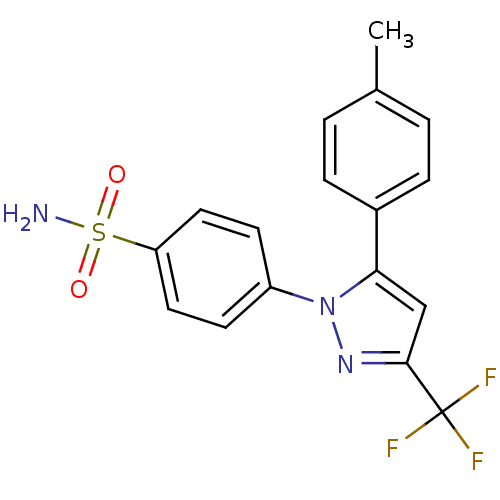
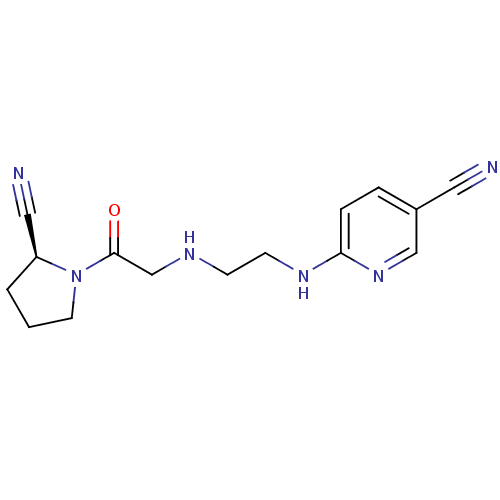
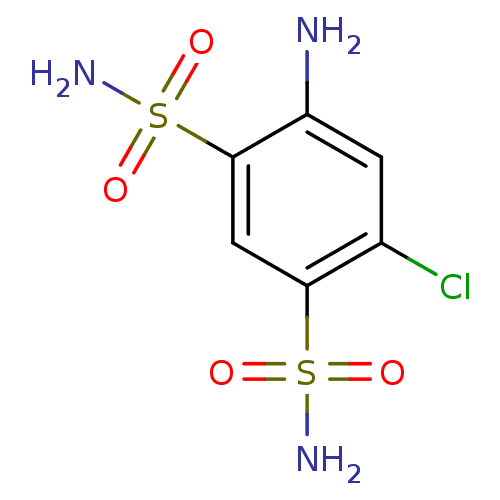
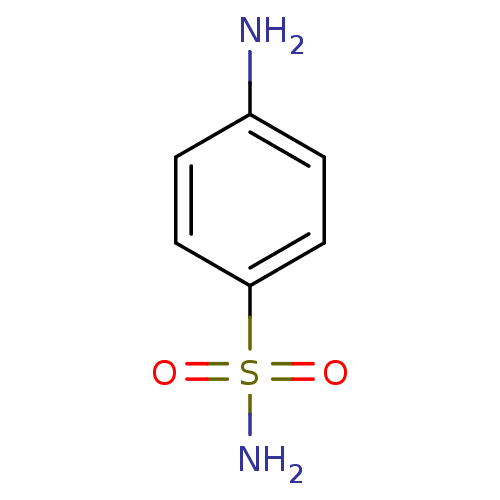
Affinity DataKi: 2.20nM ΔG°: -49.4kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nM ΔG°: -48.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 5.30nM ΔG°: -47.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
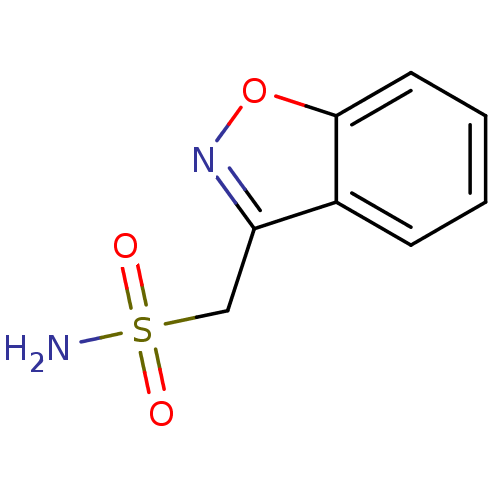
Affinity DataKi: 8nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of human wild type carbonic anhydrase 2 expressed in Escherichia coli after 15 mins preincubation by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
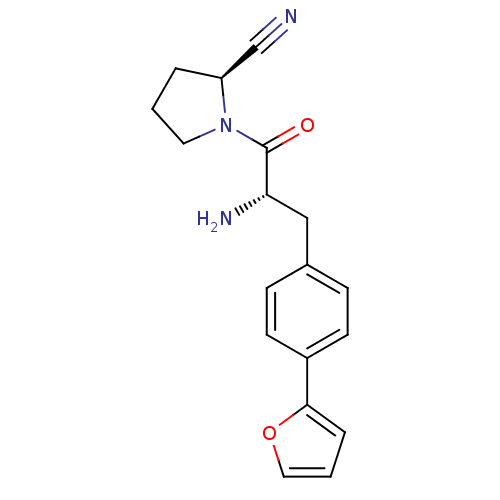
Affinity DataKi: 13nM ΔG°: -45.0kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -43.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
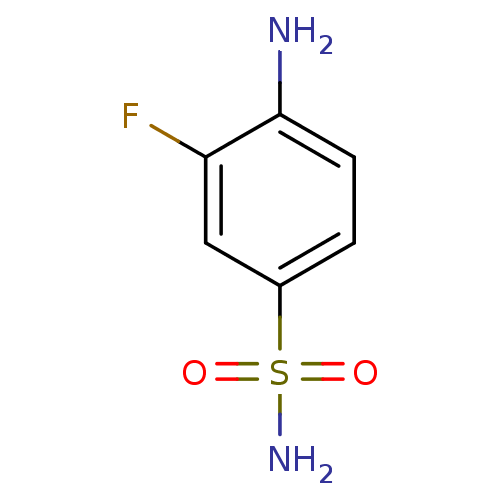
Affinity DataKi: 21nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 27nM ΔG°: -43.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 34nM ΔG°: -42.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 36nM ΔG°: -42.5kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of human wild type carbonic anhydrase 2 expressed in Escherichia coli after 15 mins preincubation by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 63nM ΔG°: -41.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Inhibition of human wild type carbonic anhydrase 2 expressed in Escherichia coli after 15 mins preincubation by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 96nM ΔG°: -40.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Inhibition of human wild type carbonic anhydrase 2 expressed in Escherichia coli after 15 mins preincubation by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 160nM ΔG°: -38.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of human wild type carbonic anhydrase 2 expressed in Escherichia coli after 15 mins preincubation by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 166nM ΔG°: -38.7kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 170nM ΔG°: -38.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:Inhibition of human wild type carbonic anhydrase 2 expressed in Escherichia coli after 15 mins preincubation by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)