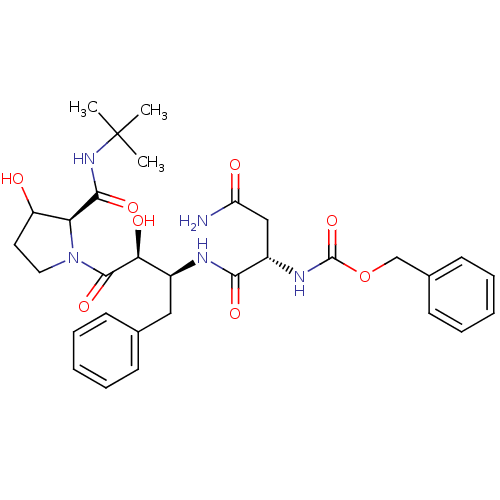
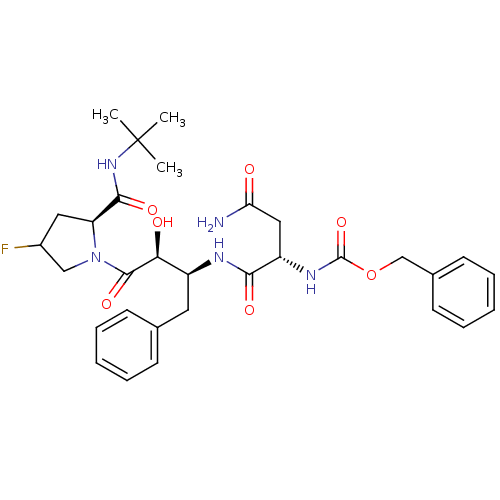
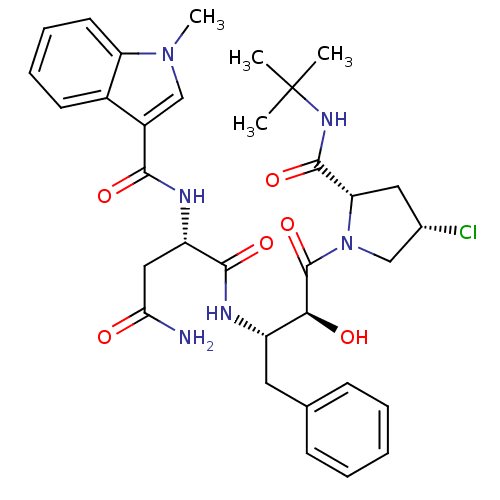
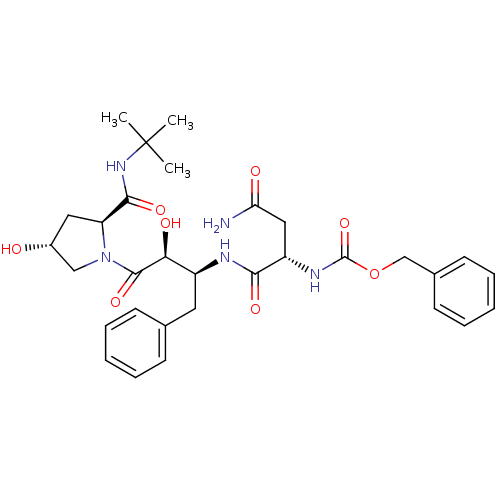
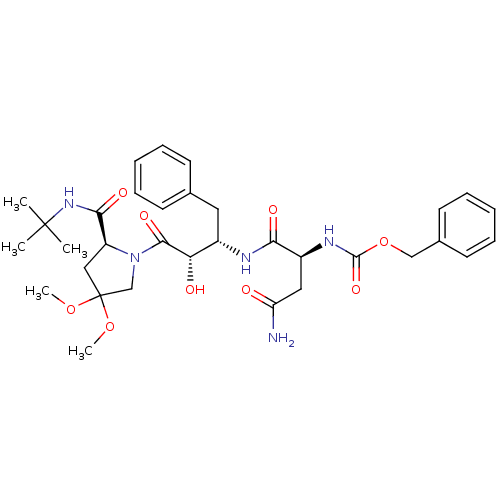
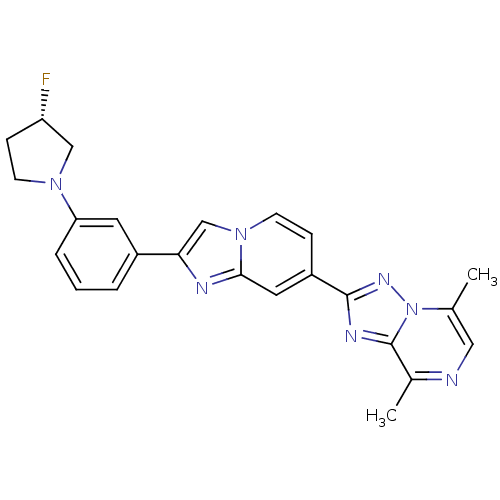
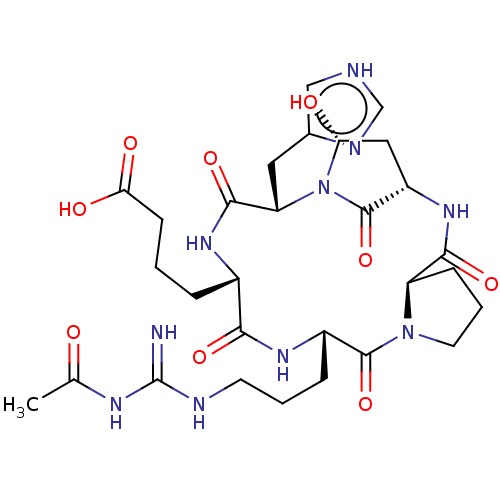
Affinity DataKi: 0.100nM ΔG°: -59.4kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
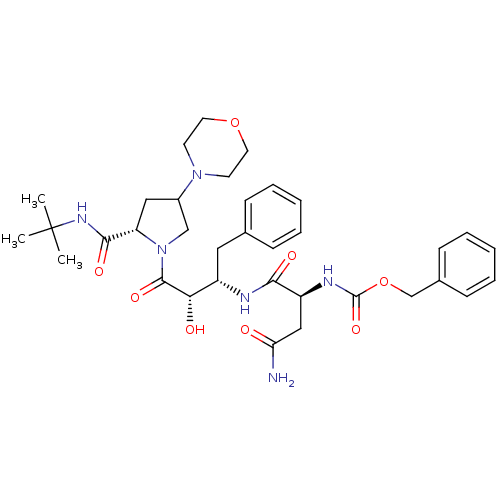
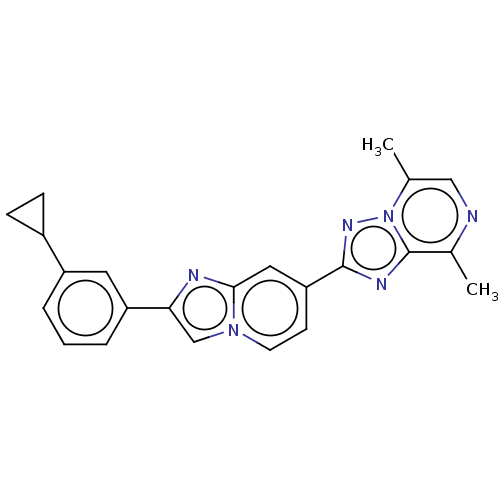
Affinity DataKi: 1nM ΔG°: -53.4kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 1nM ΔG°: -53.4kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
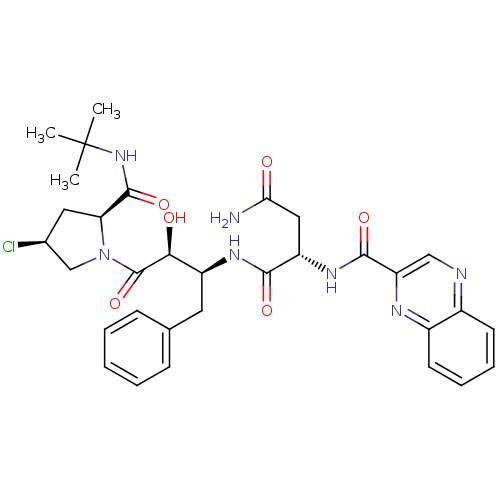
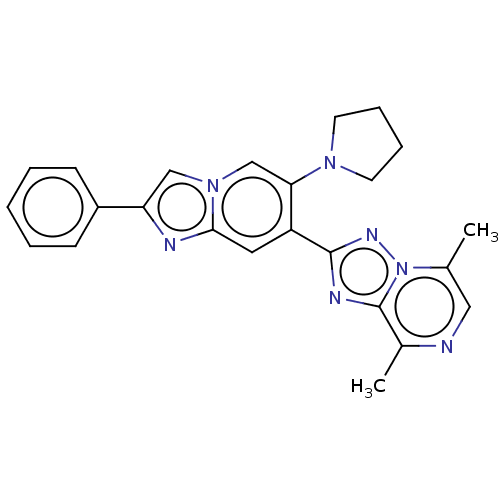
Affinity DataKi: 4.5nM ΔG°: -49.6kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
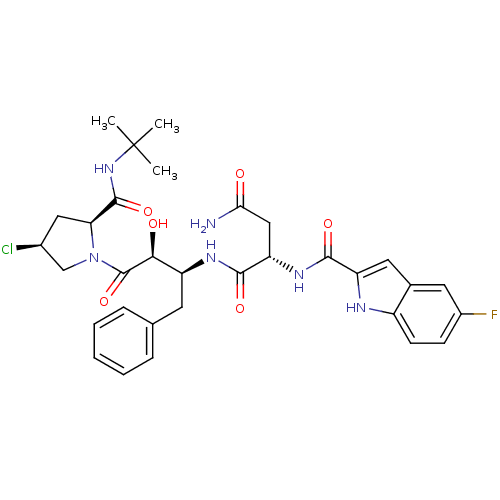
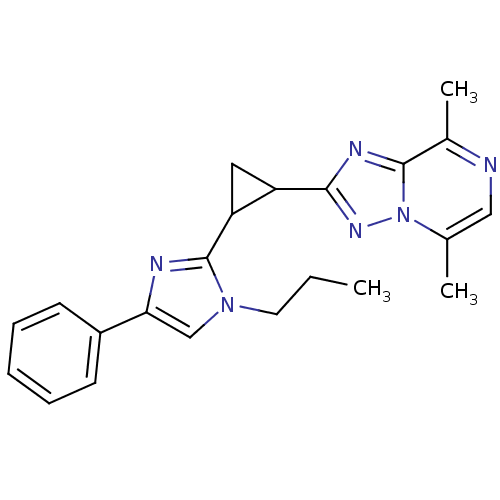
Affinity DataKi: 4.70nM ΔG°: -49.4kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 8nM ΔG°: -48.1kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 12.5nM ΔG°: -46.9kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 12.5nM ΔG°: -46.9kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 14.5nM ΔG°: -46.5kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 16nM ΔG°: -46.3kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 18nM ΔG°: -46.0kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -45.7kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 21.5nM ΔG°: -45.5kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 22.5nM ΔG°: -45.4kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 28nM ΔG°: -44.8kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 32nM ΔG°: -44.5kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 35nM ΔG°: -44.3kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 56nM ΔG°: -43.1kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 57.5nM ΔG°: -43.0kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 90nM ΔG°: -41.8kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 92nM ΔG°: -41.8kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
Affinity DataKi: 125nM ΔG°: -41.0kJ/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
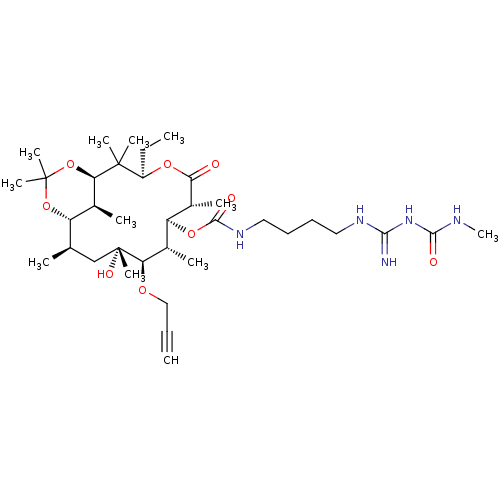
Affinity DataKi: 8.30E+4nMAssay Description:Time dependent inhibition of CYP3A4 in human liver microsomes assessed as inhibition constantMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
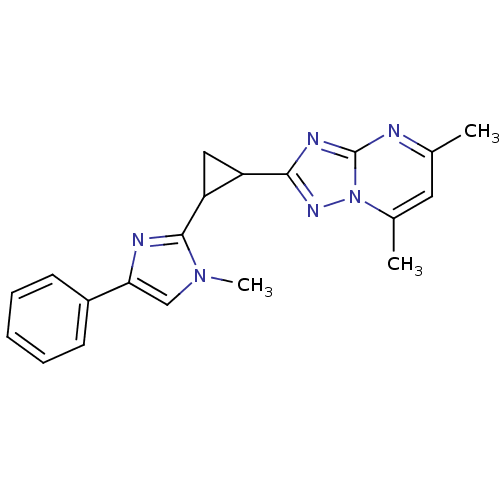
Affinity DataIC50: 0.100nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 0.160nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 0.160nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 0.330nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 1.79nMAssay Description:PDE10 activity was measured using Scintillation Proximity Assay (SPA)-based methods. PDE10 catalyses the hydrolysis of the intracellular messenger ad...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 2.40nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 3.5nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 6.65nMAssay Description:PDE10 activity was measured using Scintillation Proximity Assay (SPA)-based methods. PDE10 catalyses the hydrolysis of the intracellular messenger ad...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 11nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 14.5nMAssay Description:PDE10 activity was measured using Scintillation Proximity Assay (SPA)-based methods. PDE10 catalyses the hydrolysis of the intracellular messenger ad...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 26nMpH: 7.4Assay Description:The PDE10 inhibition assay in 384-well plates was conducted to identify substances for the inhibition of cyclic nucleotide hydrolysis by the PDE10 en...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of Serratia marcescens chitinase ChiBMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a...More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 66nMAssay Description:Inhibition of OATP1B1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of CYP3A4 in human liver microsomes measured after 30 minsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human ERG by patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 217nMAssay Description:Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a...More data for this Ligand-Target Pair