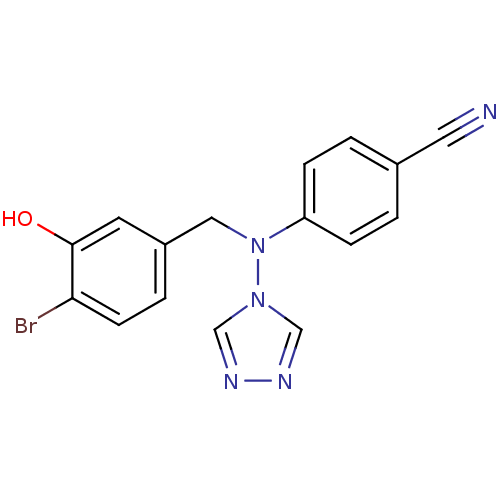
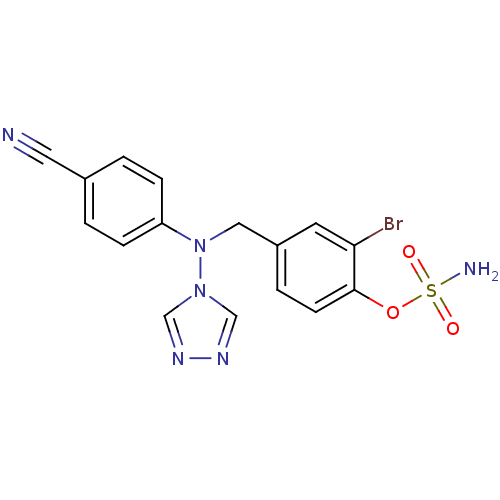
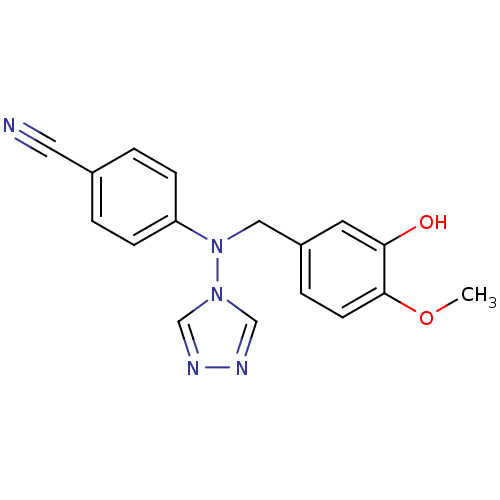
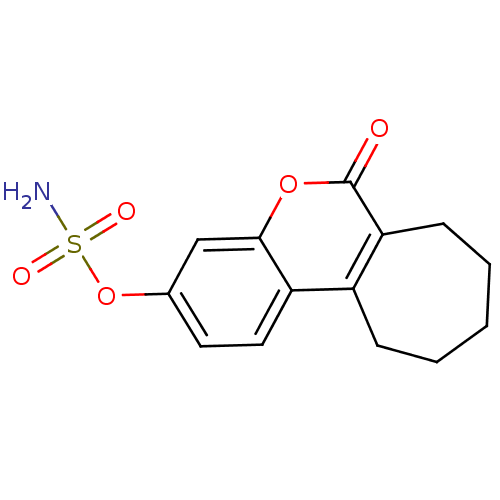
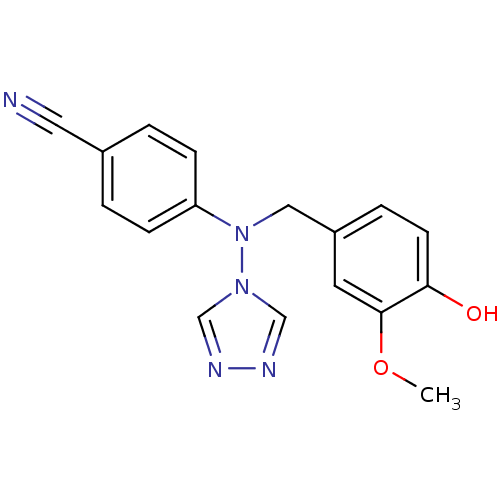
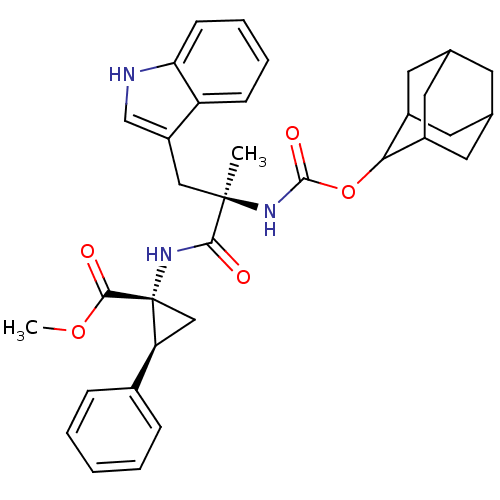
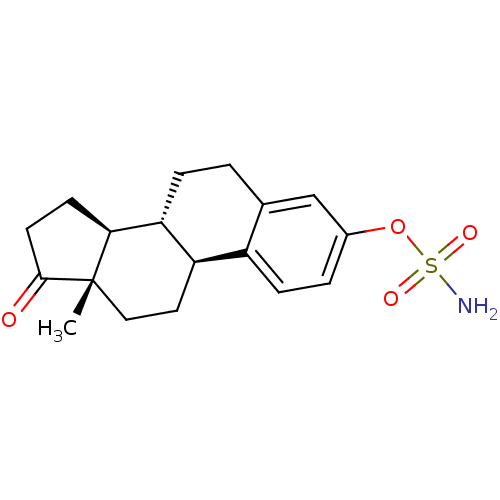
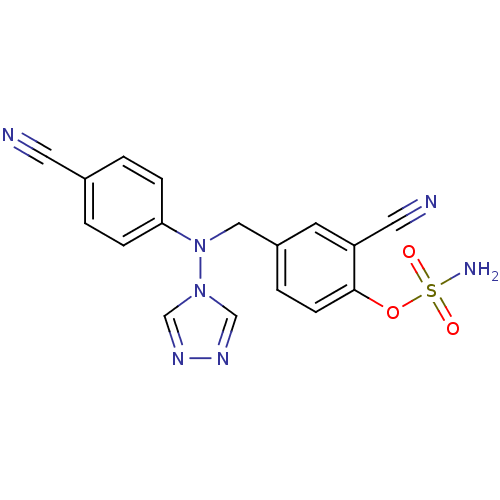
Affinity DataIC50: 0.100nMAssay Description:Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type B receptor in mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.510nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.880nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type B receptor in mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of full length human PARP-1 after 10 mins by FlashPlate scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against steroid sulfatase in placental microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibitory activity against 17 beta hydroxysteroid dehydrogenase type 1 in T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of full length human PARP-1 after 10 mins by FlashPlate scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibitory concentration against steroid sulfatase in placental microsomesMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibitory activity against 17 beta hydroxysteroid dehydrogenase type 1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1More data for this Ligand-Target Pair
Affinity DataIC50: 39nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of steroid sulfatase in placental microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMpH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibitory concentration against steroid sulfatase in placental microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type B receptor in mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Compound was tested for the inhibition of specific binding of [125I]-Bolton Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreasMore data for this Ligand-Target Pair