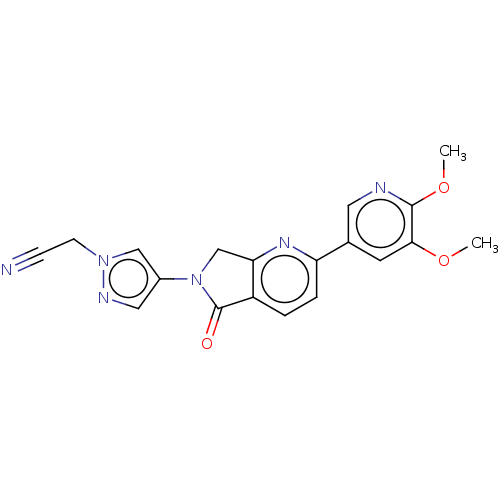
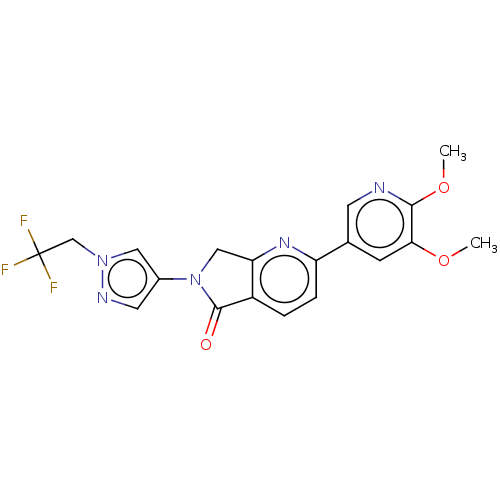
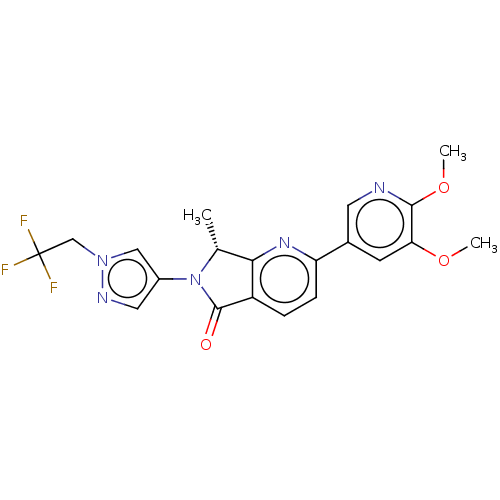
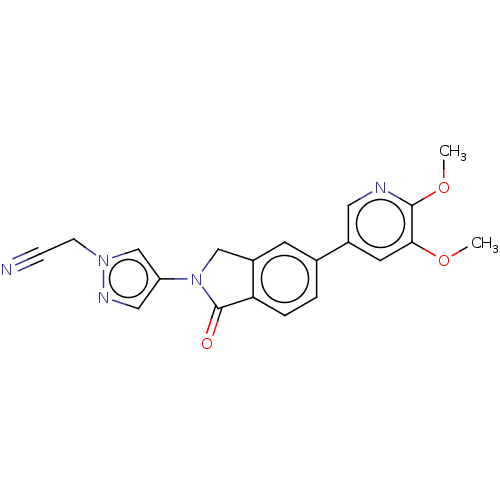
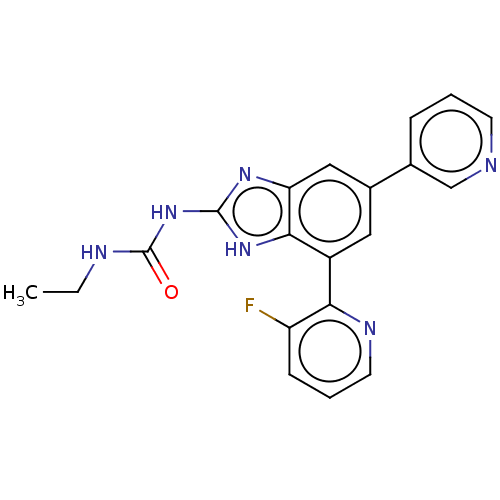
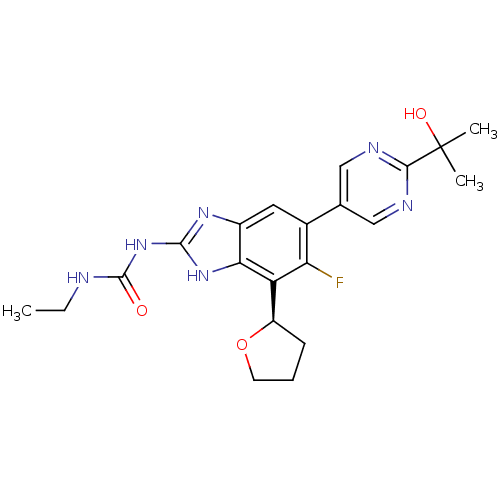
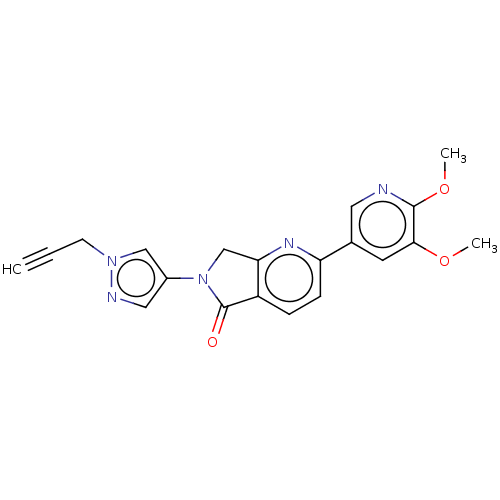
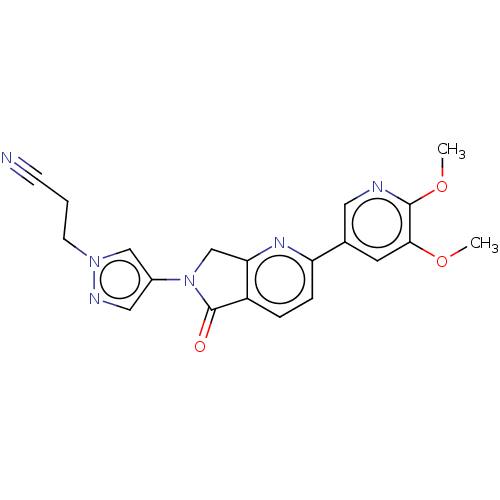
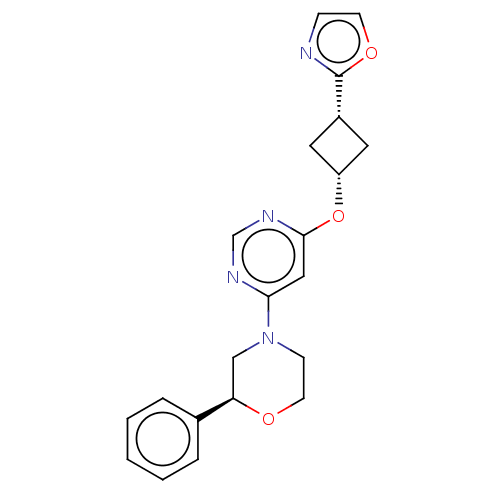
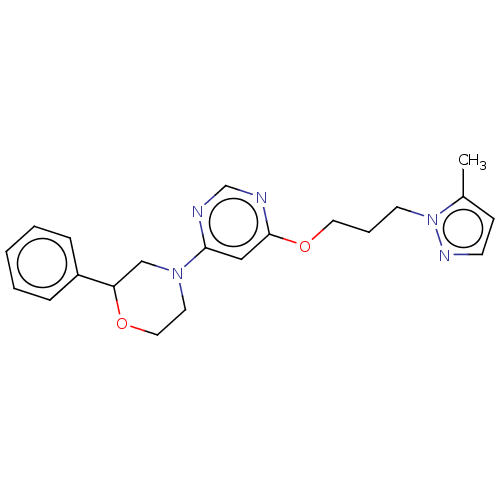
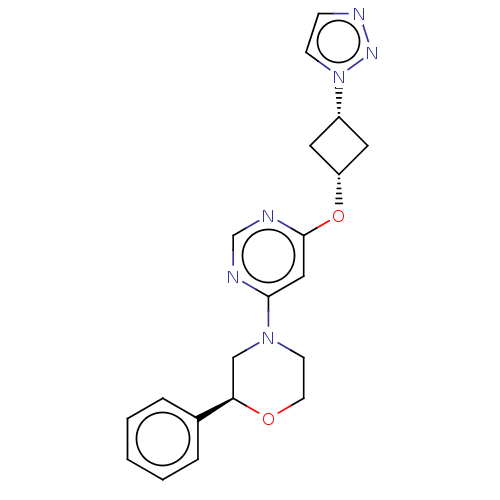
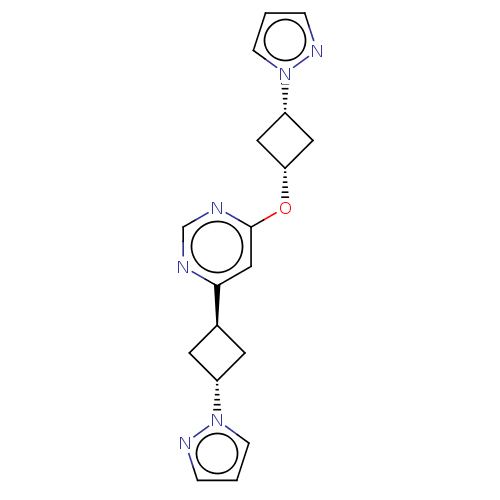
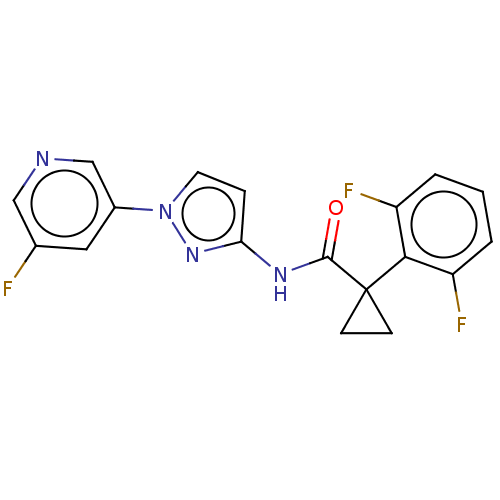
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]ATP by scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: <4nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase using pBR322 plasmid DNA as substrate by coupled enzyme reaction assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]ATP by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: <17.7nMAssay Description:Inhibition of Staphylococcus aureus DNA gyraseMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: <21nMAssay Description:Inhibition of Staphylococcus aureus DNA gyraseMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibition of Staphylococcus aureus DNA topoisomerase 4More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Inhibition of Staphylococcus aureus DNA topoisomerase 4More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDNA-dependent protein kinase catalytic subunit(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Inhibition of DNA-PK (unknown origin) using EPPLSQEAFADLWKKK as substrate after 15 mins in presence of [33P-ATP] by radiometric methodMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of ELOVL1 in Het Female 1 patient-derived human lymphocyte assessed as reduction of C26:0 VLCFA synthesis incubated for 48 hrs by cellular...More data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of ELOVL1 in Het Female 2 patient-derived human lymphocyte assessed as reduction of C26:0 VLCFA synthesis incubated for 48 hrs by cellular...More data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of ELOVL1 in human microglia assessed as reduction of C26:0 VLCFA synthesis incubated for 48 hrs by cellular assayMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of ELOVL1 in human HEK2936E cells assessed as reduction of C26:0 LPC synthesis incubated for 48 hrs by cellular assayMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of ELOVL1 in human HEK293 cells assessed as reduction of C26:0 VLCFA synthesis incubated for 48 hrs by cellular assayMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of ELOVL1 in human HEK2936E cells assessed as reduction of C26:0 LPC synthesis incubated for 48 hrs by cellular assayMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of ELOVL1 in HEK293 cells assessed as reduction of C26:0 lipo phosphatidyl choline synthesisMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of ELOVL1 in CALD patient-derived human lymphocyte assessed as reduction of C26:0 VLCFA synthesis incubated for 48 hrs by cellular assayMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of ELOVL1 in human HEK2936E cells assessed as reduction of C26:0 LPC synthesis incubated for 48 hrs by cellular assayMore data for this Ligand-Target Pair
TargetElongation of very long chain fatty acids protein 1(Homo sapiens (Human))
Vertex Pharmaceuticals
Curated by ChEMBL
Vertex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of ELOVL1 in AMN 2 patient-derived fibroblast assessed as reduction of C26:0 VLCFA synthesis incubated for 48 hrs by cellular assayMore data for this Ligand-Target Pair

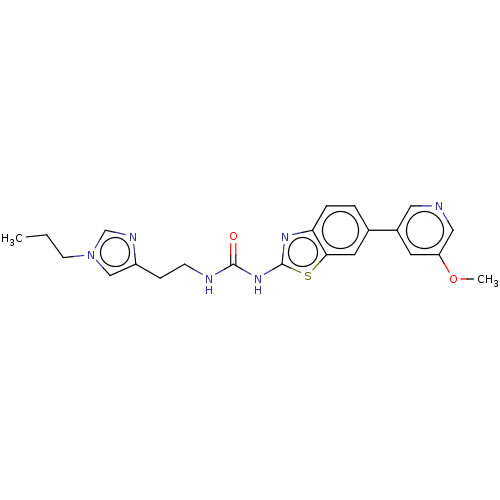
 3D Structure (crystal)
3D Structure (crystal)