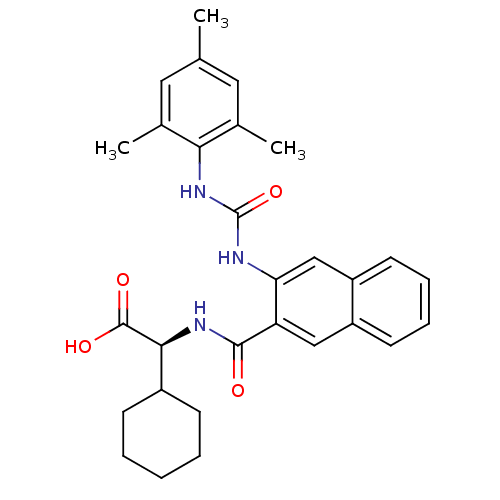
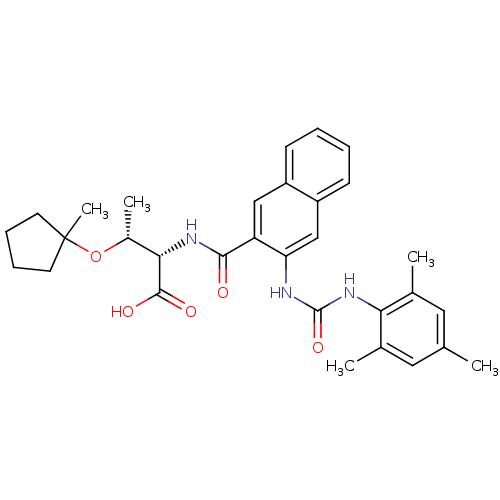
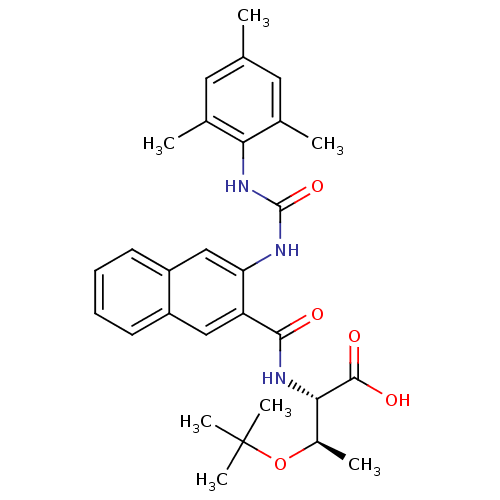
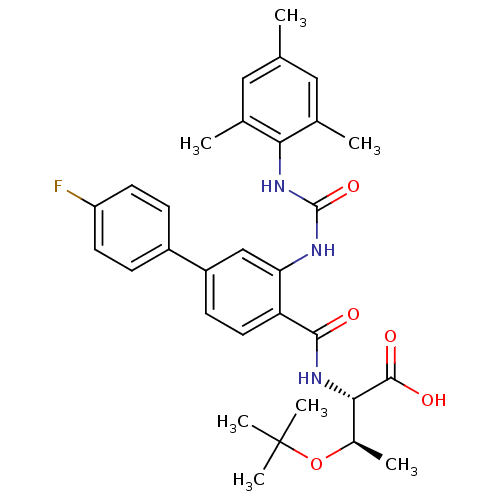
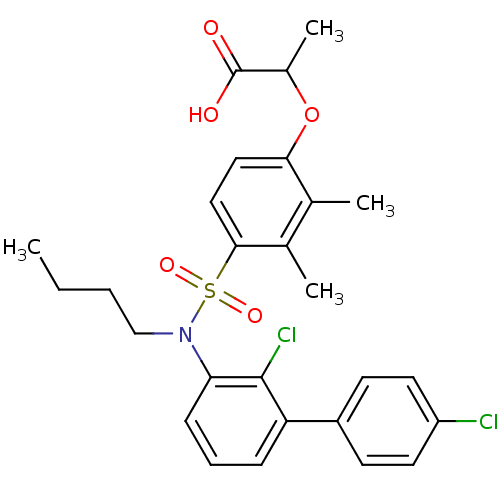
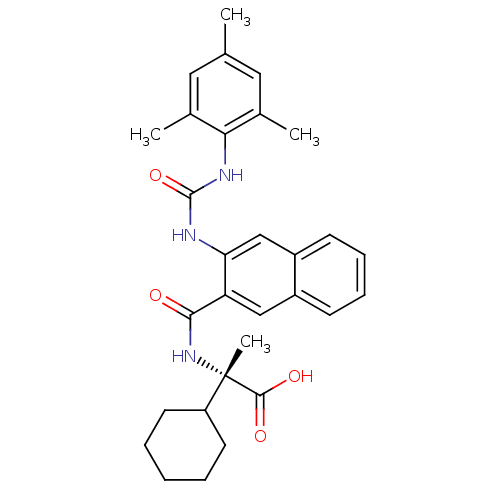
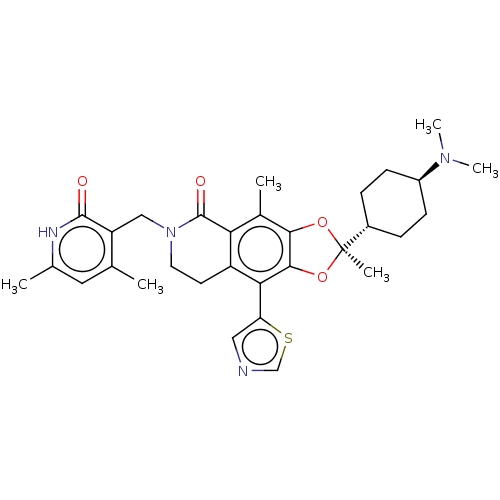
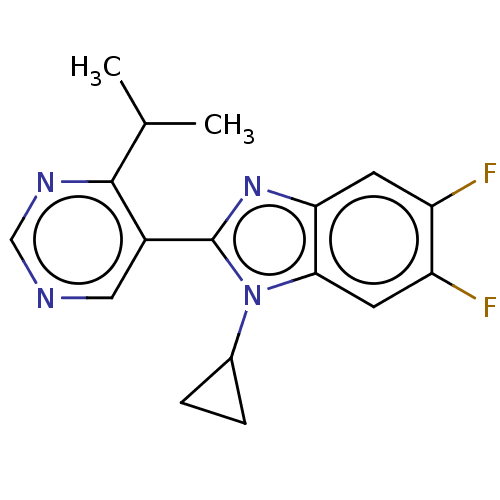
Affinity DataIC50: 0.350nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 0.360nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 0.590nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.20nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.21nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 1.36nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand InfoPurchase
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
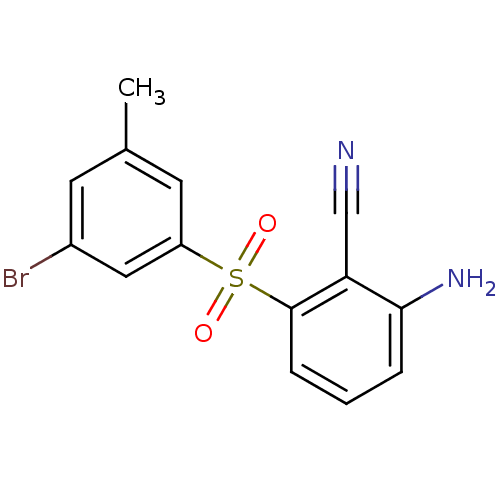
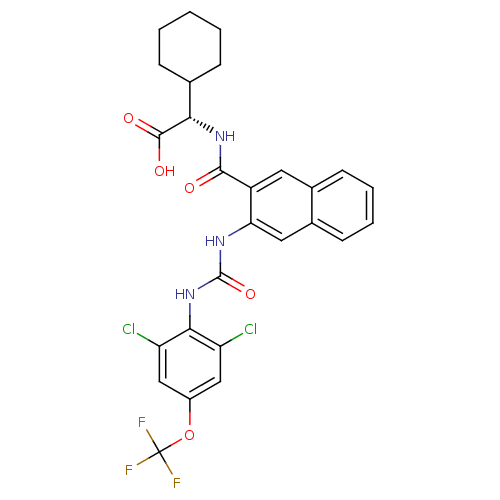
Affinity DataIC50: <2nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 2.45nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 2.70nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
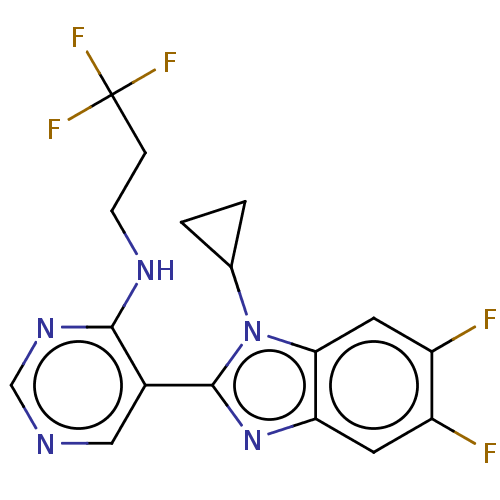
Affinity DataIC50: 3nMAssay Description:The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 3.30nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 3.40nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 3.80nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 4nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucoseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucoseMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
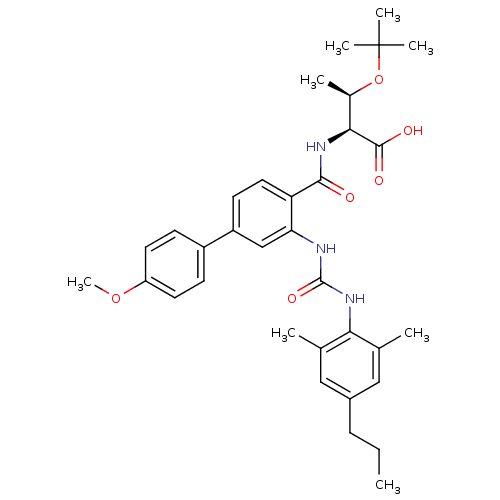
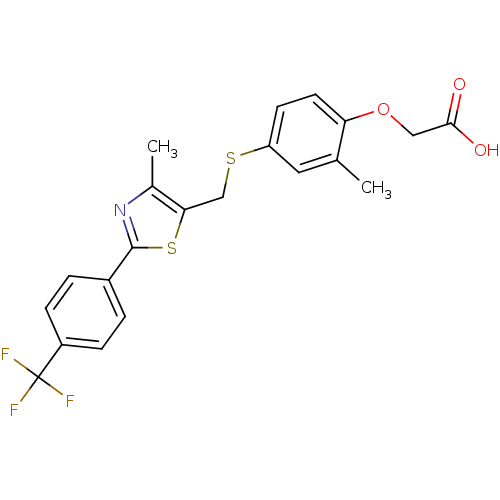
Affinity DataIC50: 5nMAssay Description:Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucoseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of mouse FFA4 receptor expressed in U2OS cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of rat FFA4 receptor expressed in U2OS cellsMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 8.31nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 9nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assayMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucoseMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 12nMAssay Description:The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea...More data for this Ligand-Target Pair
Ligand Info
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair






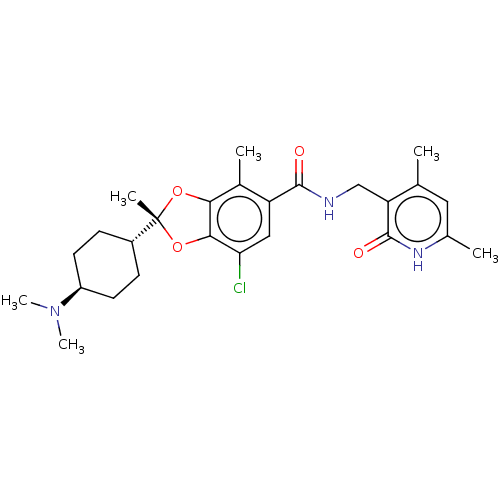


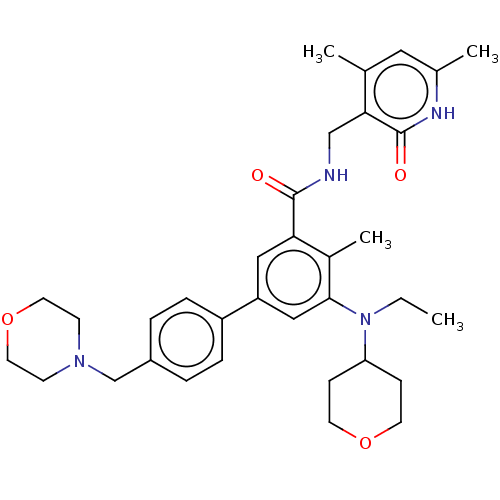











 3D Structure (crystal)
3D Structure (crystal)