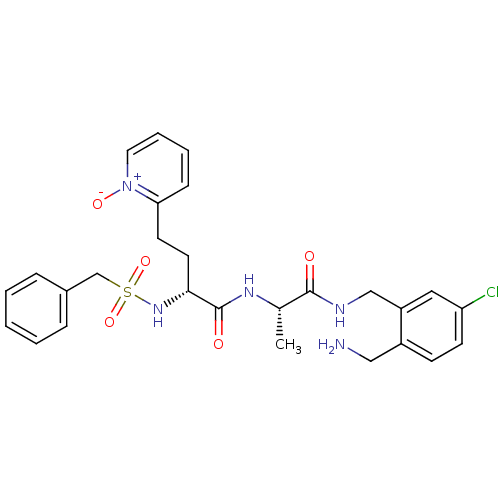
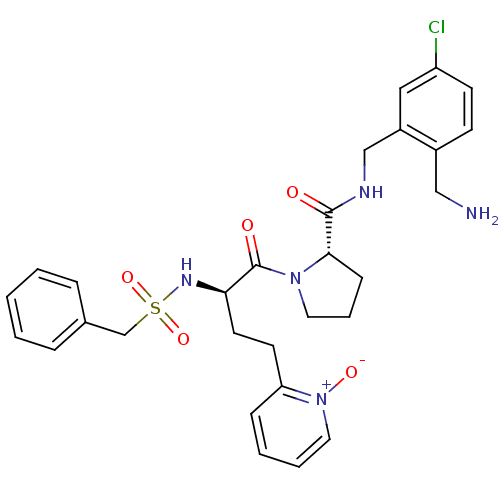
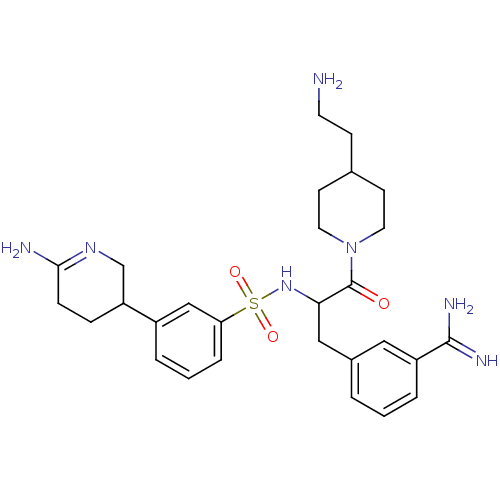
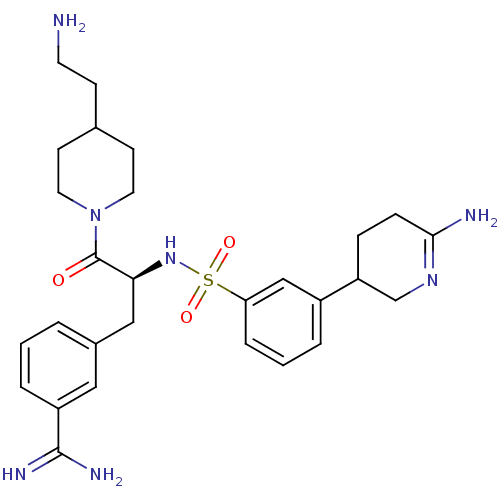
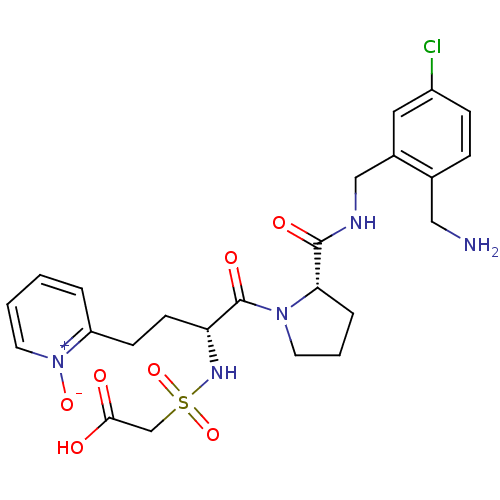
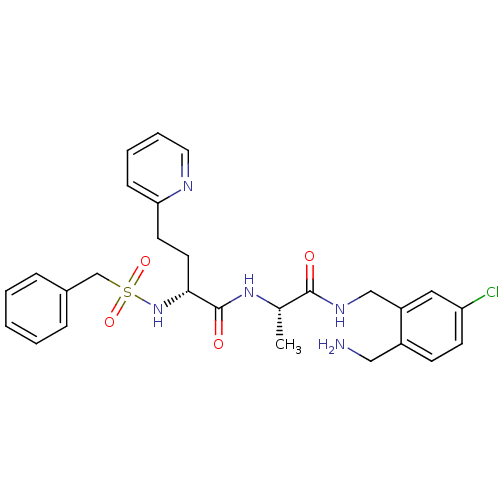
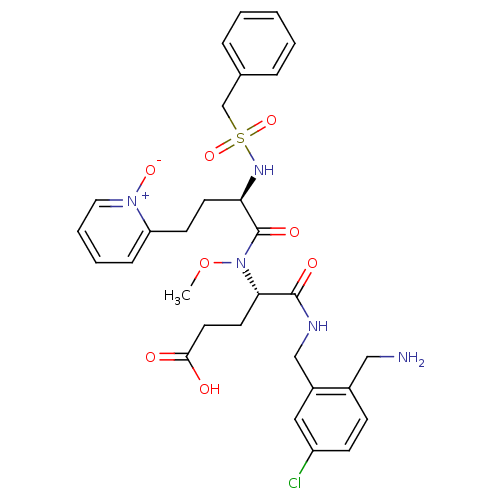
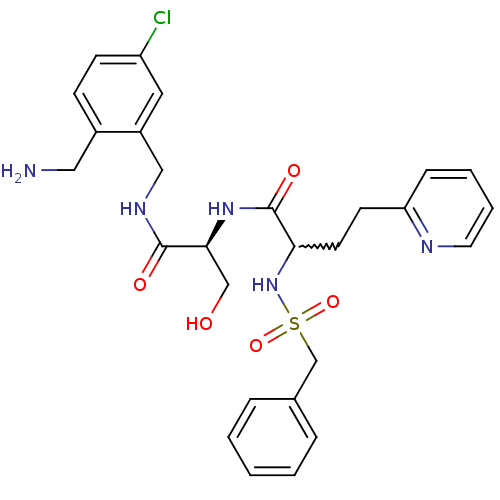
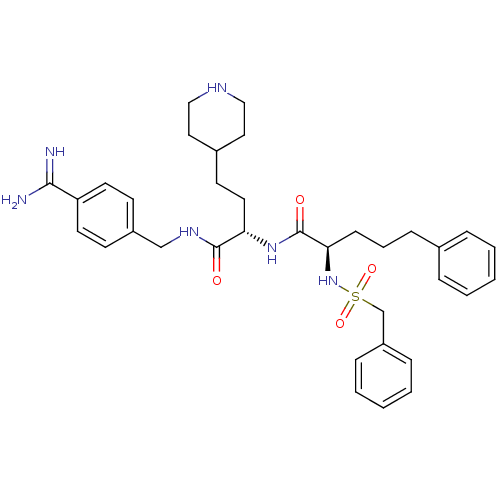
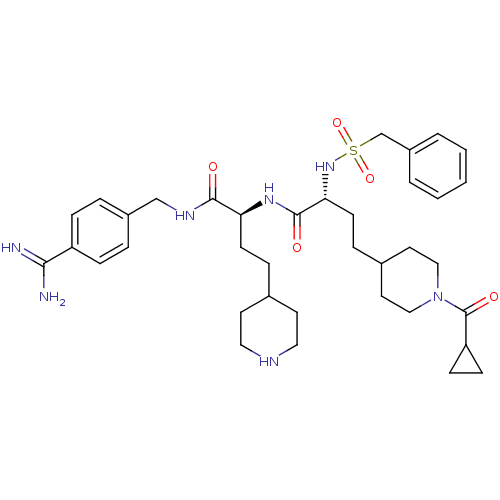
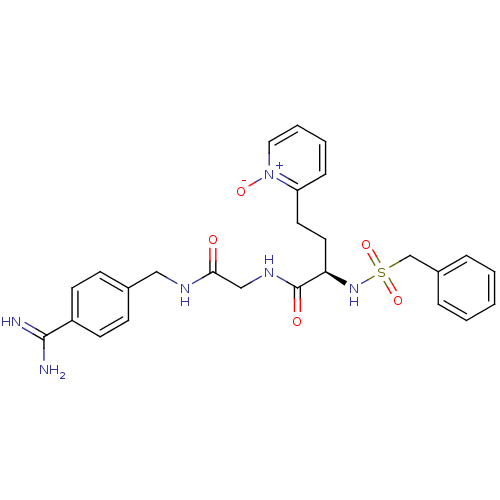
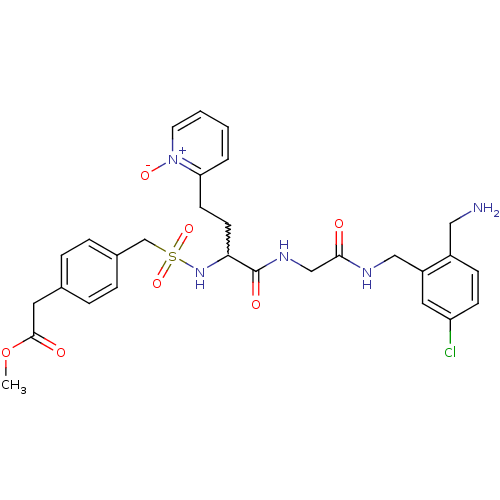
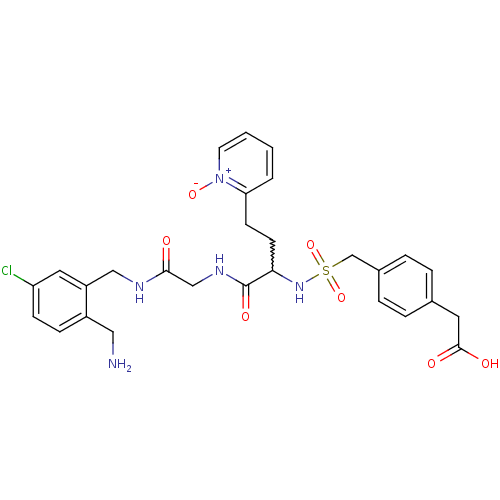
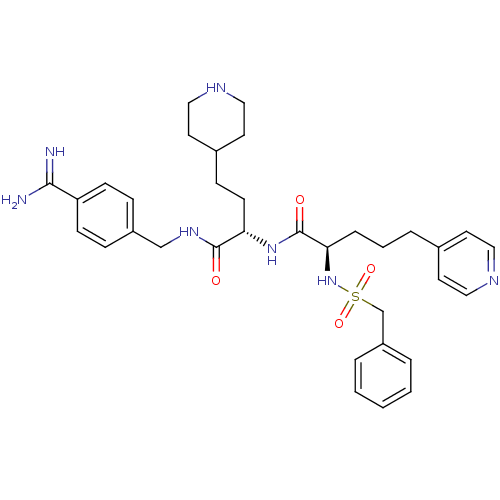
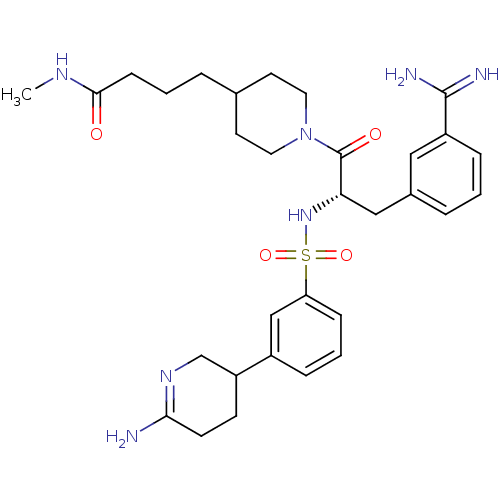
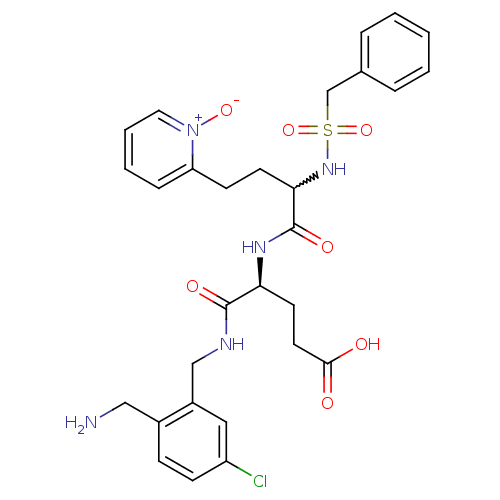
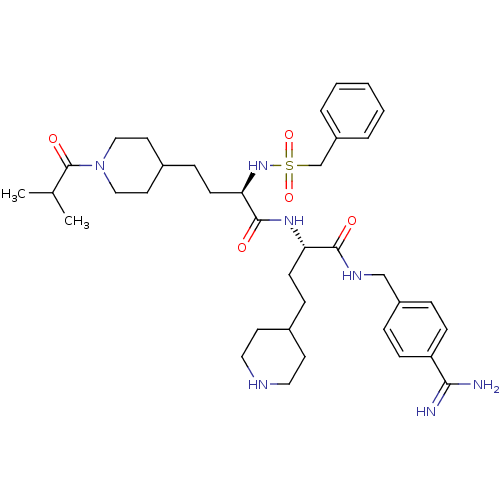
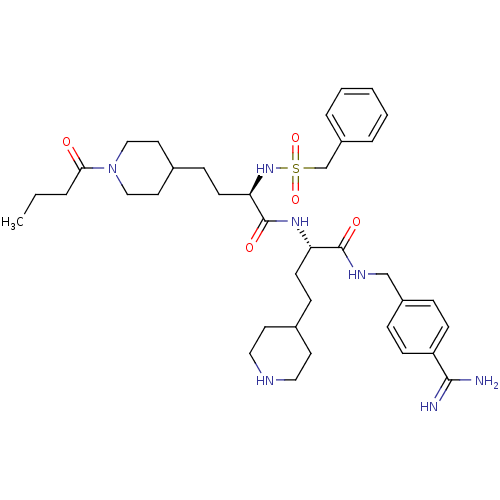
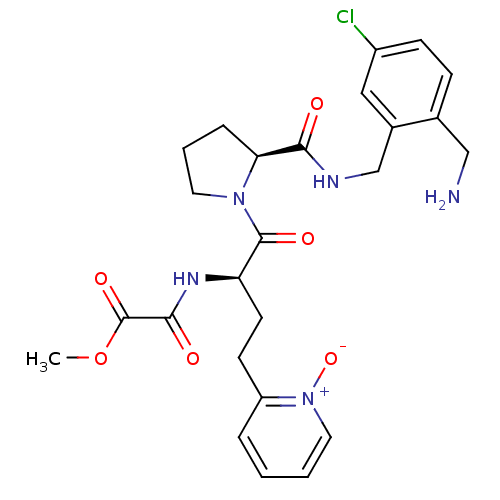
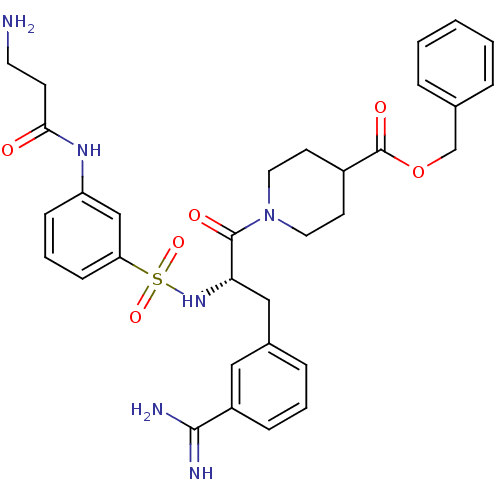
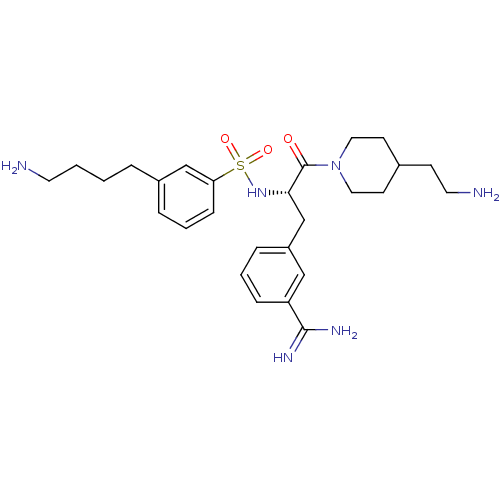
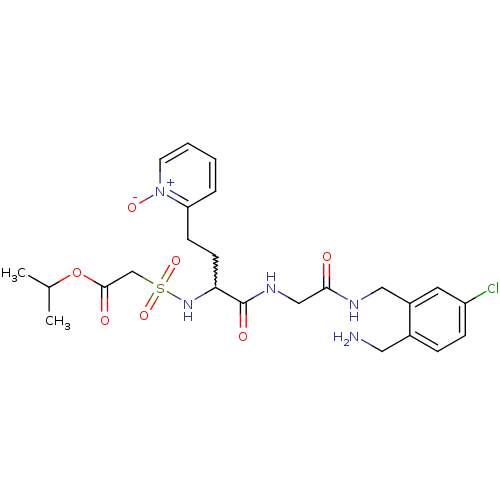
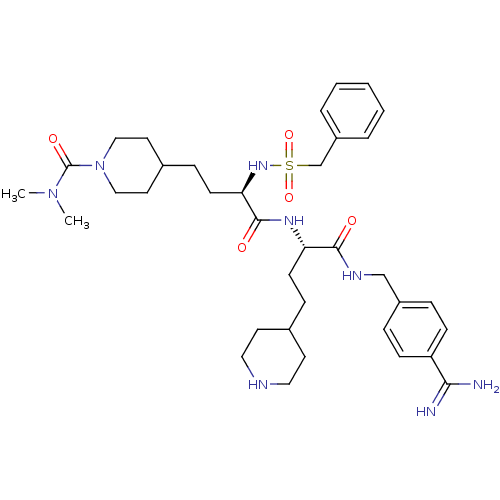
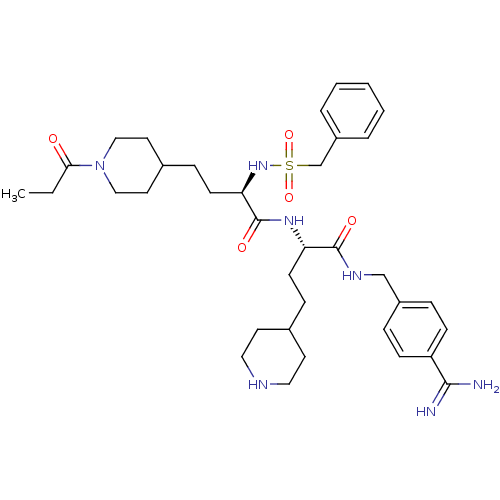
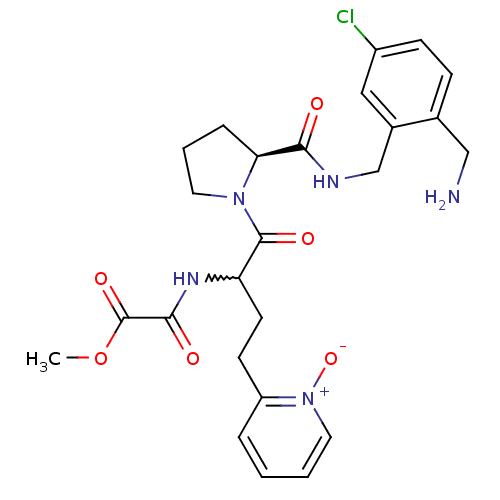
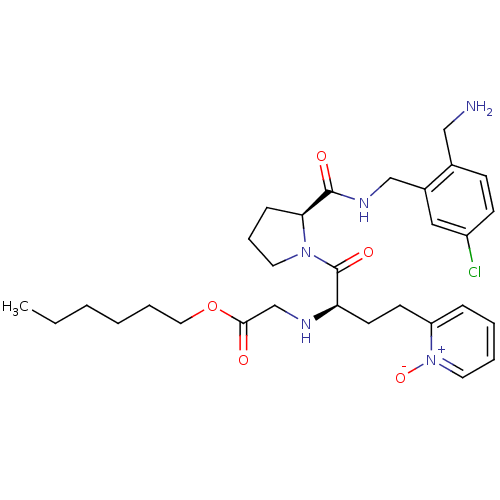
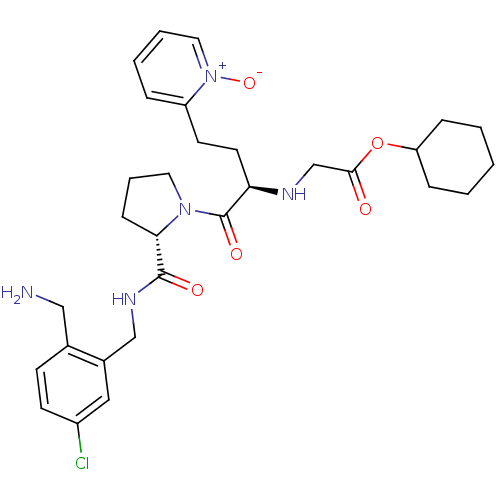
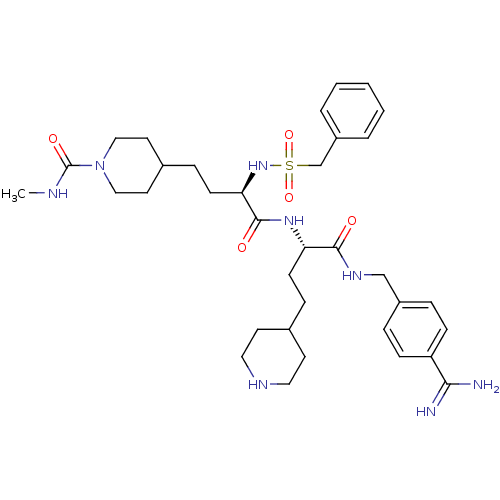
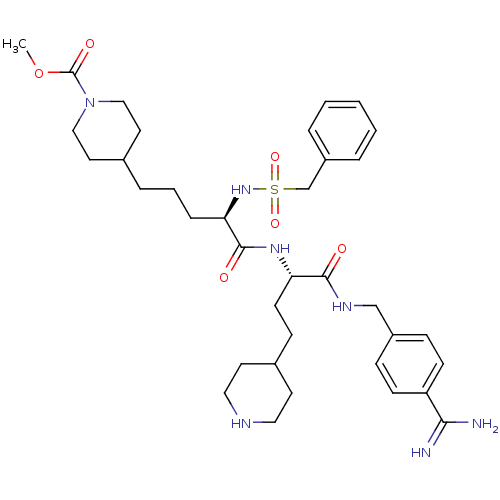
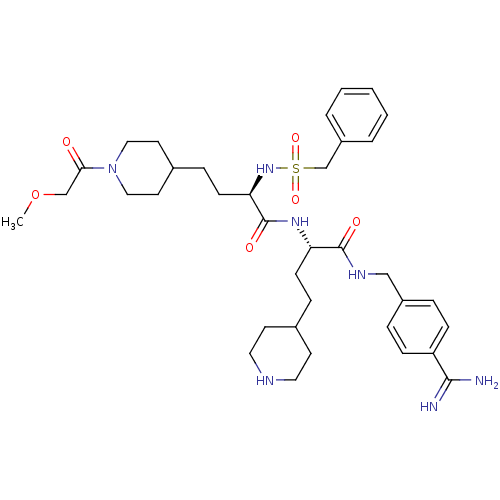
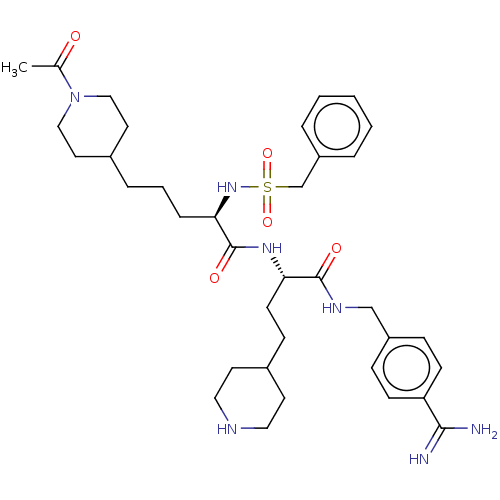
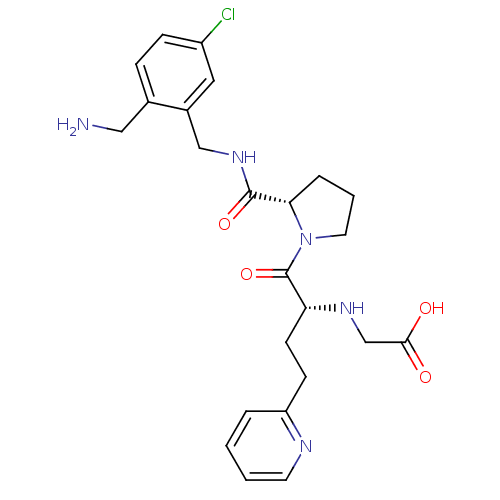
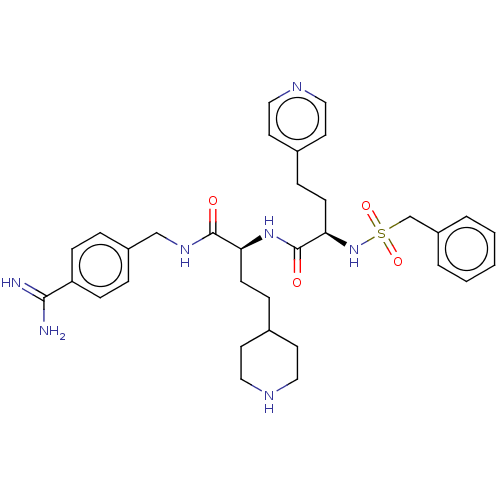
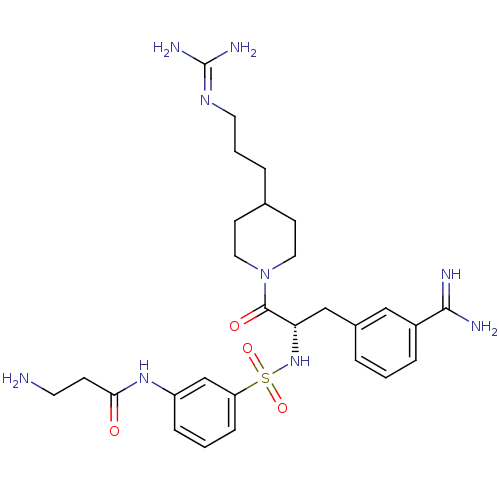
Affinity DataKi: 0.0330nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.0490nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.0590nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
The Medicines Company (Leipzig)
US Patent
The Medicines Company (Leipzig)
US Patent
Affinity DataKi: 0.0690nMAssay Description:Inhibition assay using matriptase enzymeMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
The Medicines Company (Leipzig)
US Patent
The Medicines Company (Leipzig)
US Patent
Affinity DataKi: 0.0800nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.0840nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.0950nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.320nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.350nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.360nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
The Medicines Company (Leipzig)
US Patent
The Medicines Company (Leipzig)
US Patent
Affinity DataKi: 0.490nMAssay Description:Inhibition assay using matriptase enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 0.520nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.560nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
The Medicines Company (Leipzig)
US Patent
The Medicines Company (Leipzig)
US Patent
Affinity DataKi: 0.700nMAssay Description:Inhibition assay using matriptase enzymeMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
The Medicines Company (Leipzig)
US Patent
The Medicines Company (Leipzig)
US Patent
Affinity DataKi: 0.740nMAssay Description:Inhibition assay using matriptase enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 0.760nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 0.920nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.940nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
The Medicines Company (Leipzig)
US Patent
The Medicines Company (Leipzig)
US Patent
Affinity DataKi: 1nMAssay Description:Inhibition of matriptase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:The inhibitory effect for the individual enzymes was determined in analogy to a previously disclosed method (Stürzebecher et al., J. Med. Chem.,...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
The Medicines Company (Leipzig)
US Patent
The Medicines Company (Leipzig)
US Patent
Affinity DataKi: 1.5nMAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair