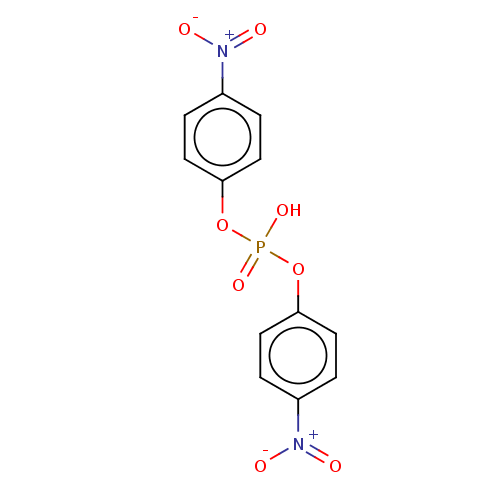
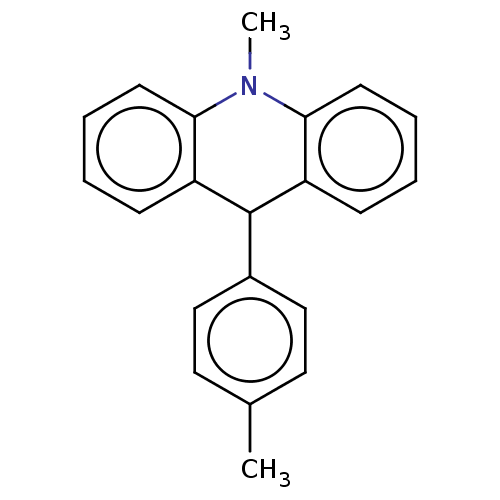
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 350nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 410nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 490nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 940nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 1.46E+3nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 2.66E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 3.34E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 3.75E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 4.01E+3nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 6.43E+3nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 1.32E+4nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 1.90E+4nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 810nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 840nMAssay Description:Negative allosteric modulation of rat mGlu5 receptor expressed in HEK293 cells assessed as inhibition of glutamate induced-calcium mobilization prein...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.08E+3nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.17E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.74E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.35E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.83E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 7.62E+3nMAssay Description:Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.18E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.23E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.24E+4nMAssay Description:Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.37E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.57E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.67E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.88E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth...More data for this Ligand-Target Pair

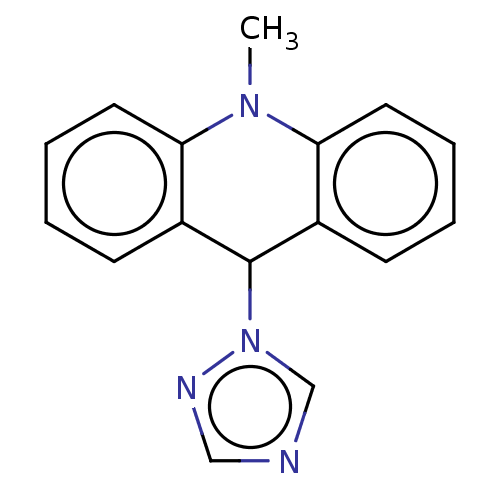
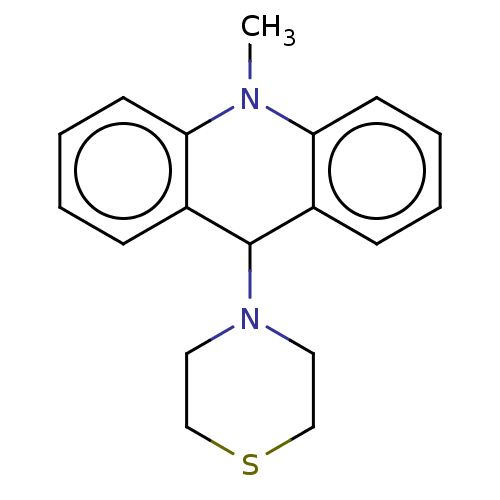
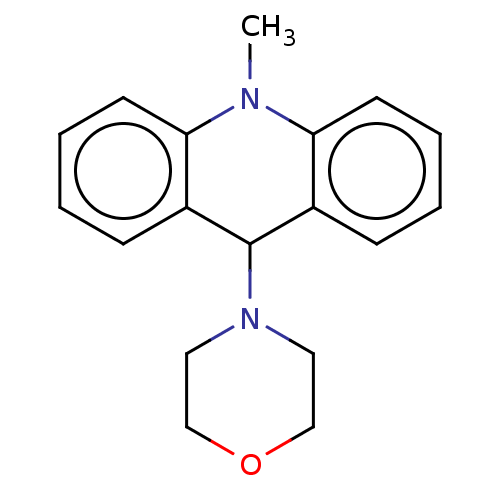

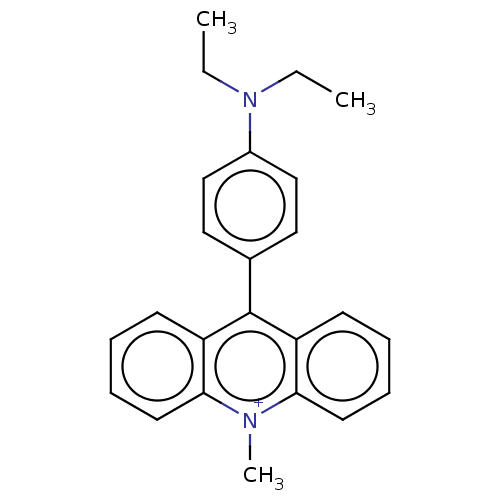
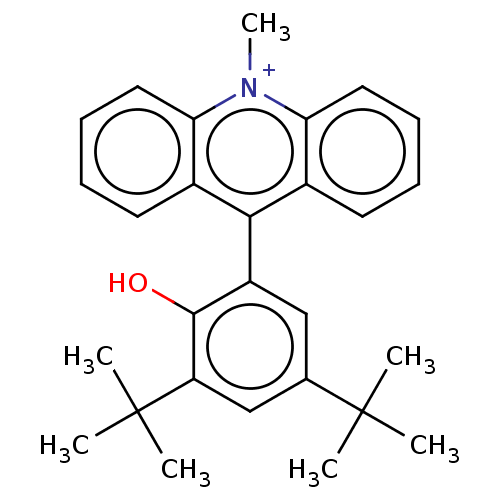

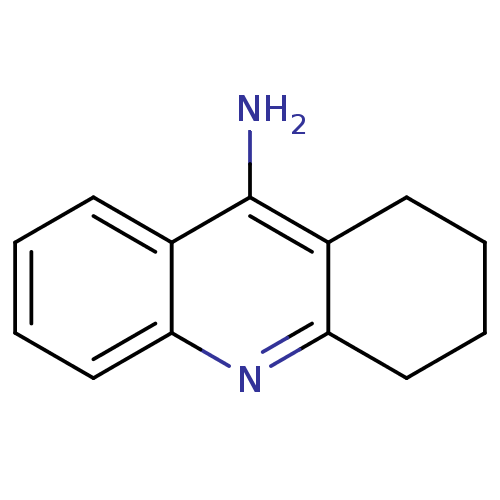
 3D Structure (crystal)
3D Structure (crystal)