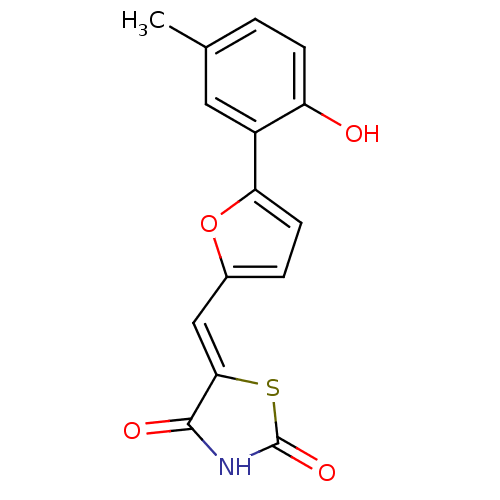
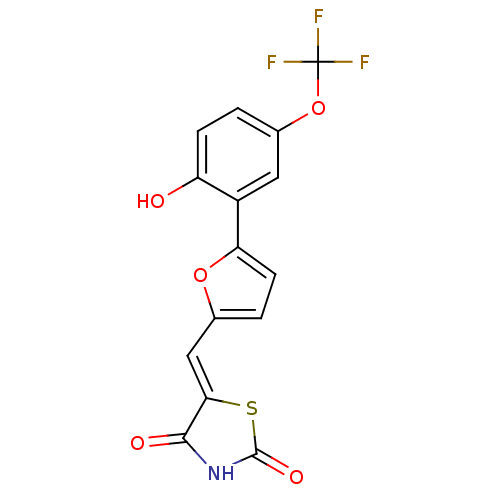
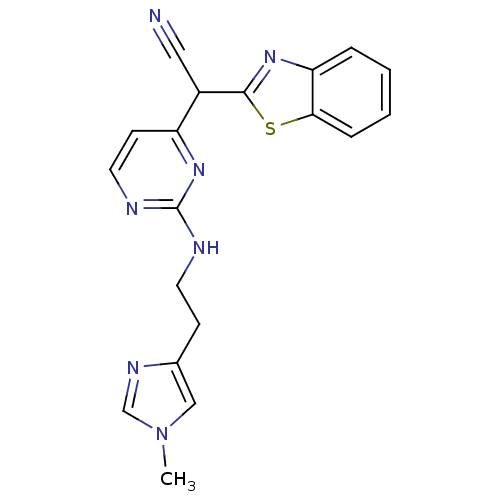
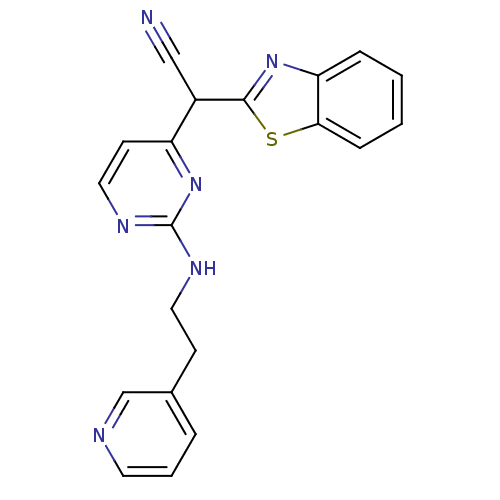
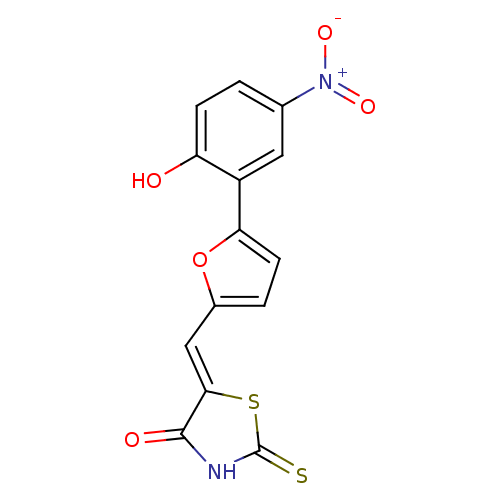
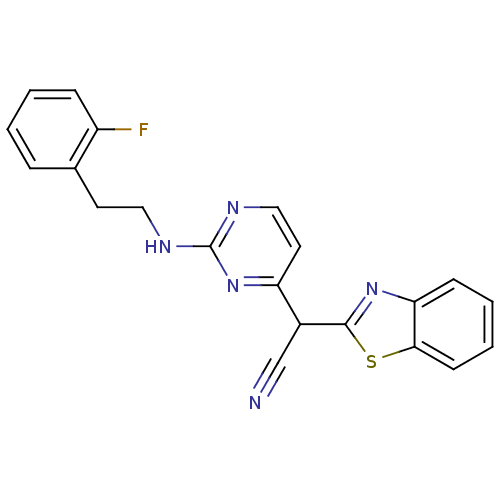
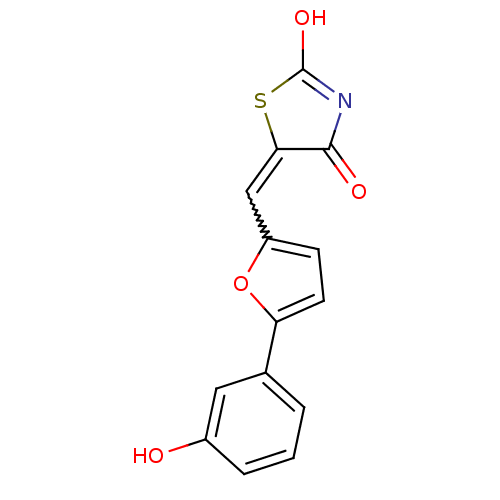
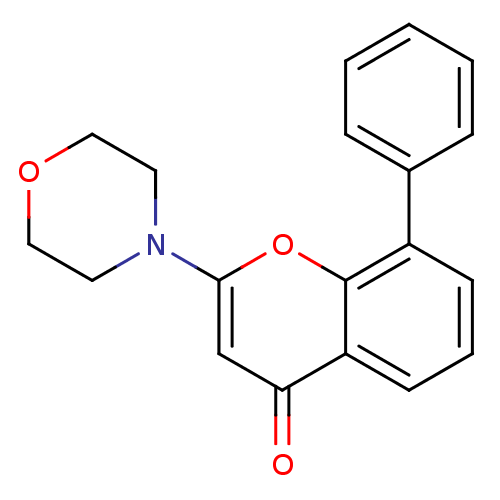
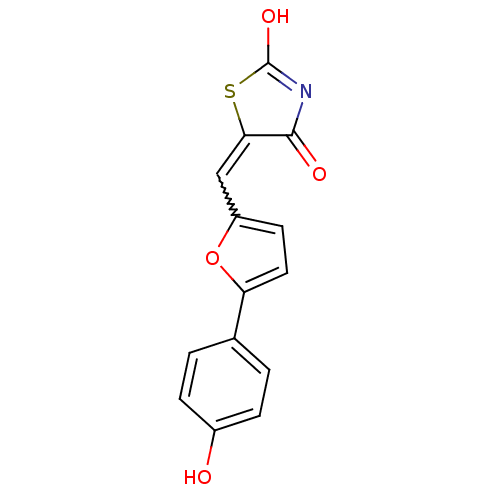
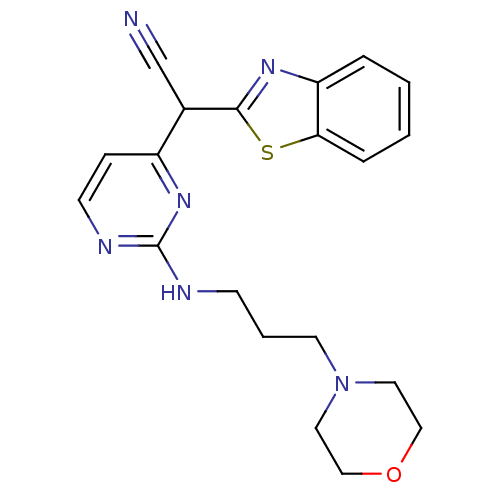
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human PI3KdeltaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human PI3KbetaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of c-Jun N-Terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 143nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 147nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 8(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of c-Jun N-Terminal kinase 1More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 185nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibitory concentration against c-Jun N-Terminal kinase 2More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 273nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 306nMAssay Description:Inhibition of human PI3KbetaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 337nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 397nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 407nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 455nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 458nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Rattus norvegicus)
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 473nMAssay Description:Inhibition of rat c-Jun N-terminal kinase 3More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)