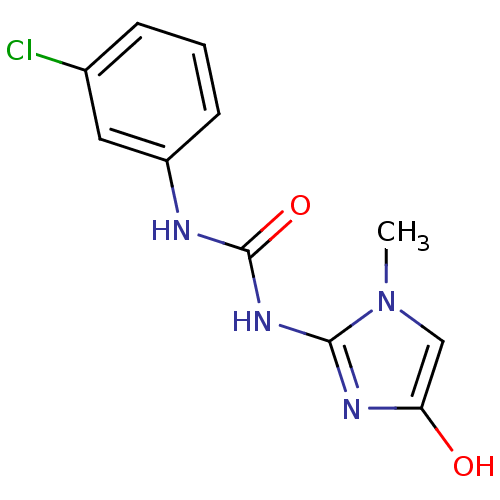
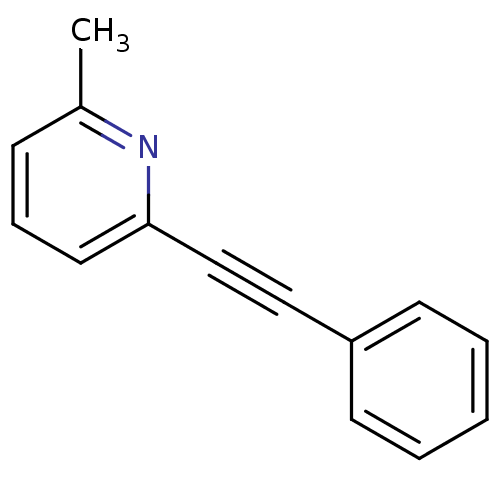
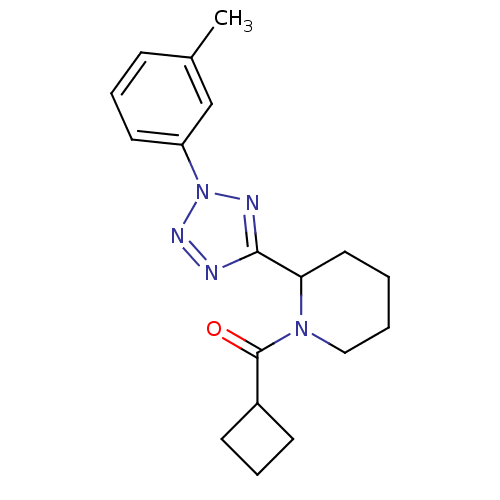
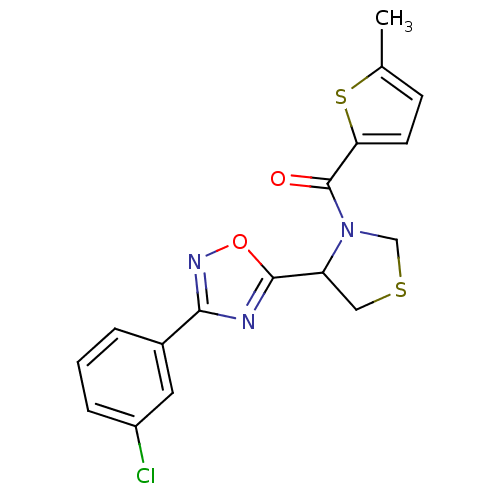
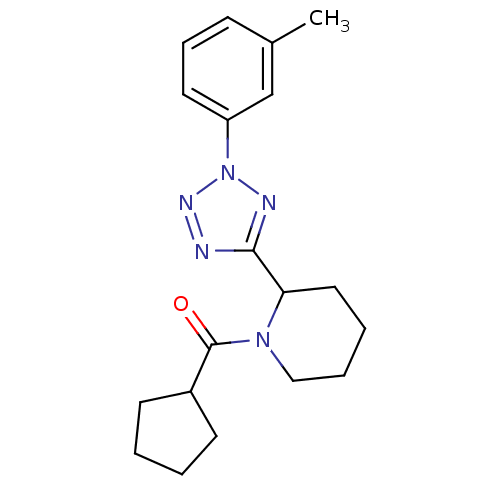
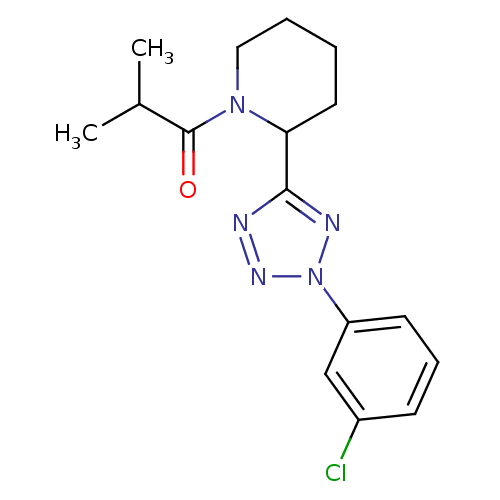
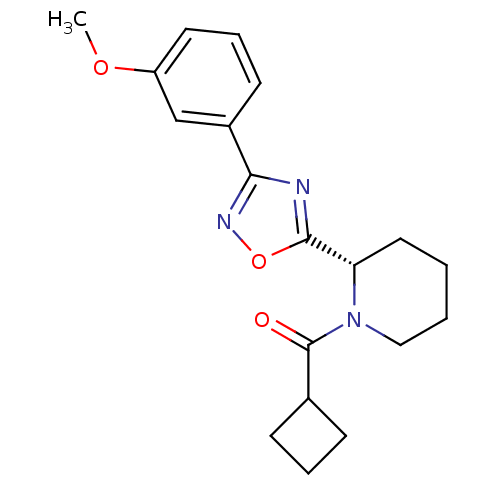
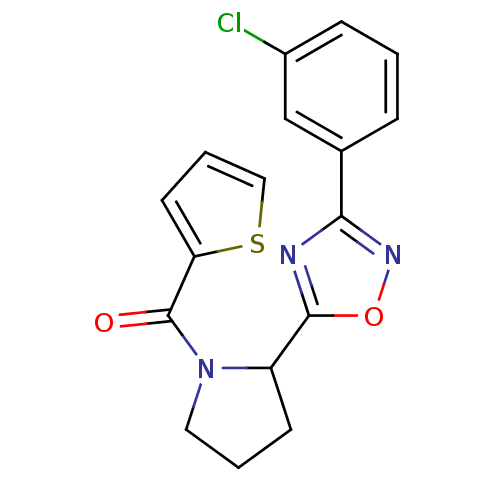
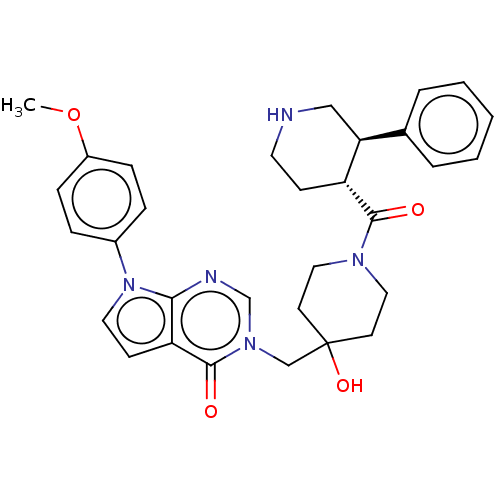
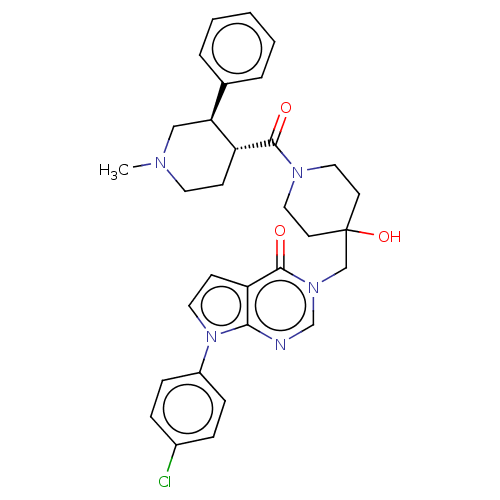
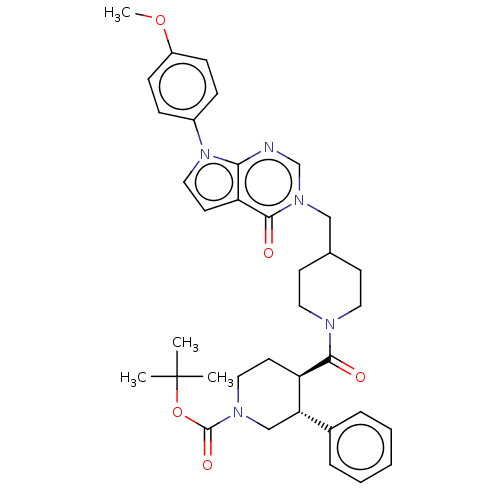
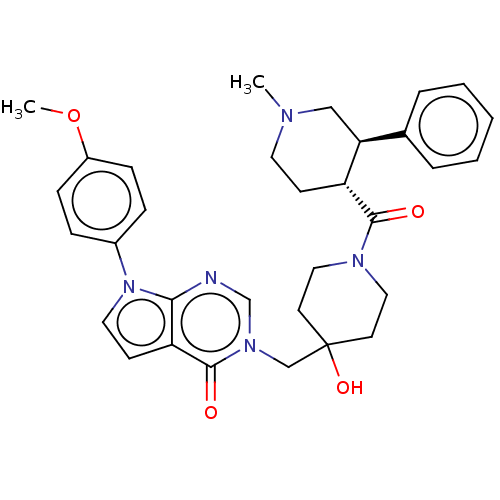
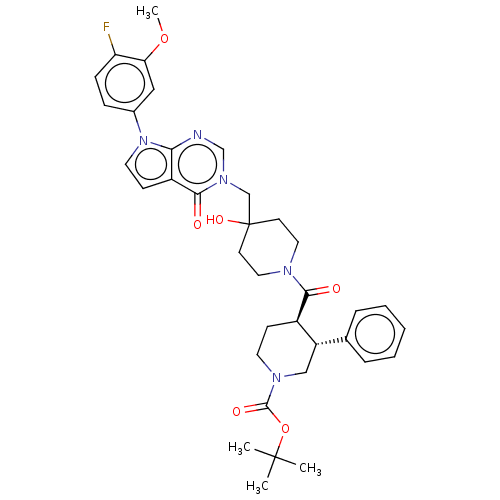
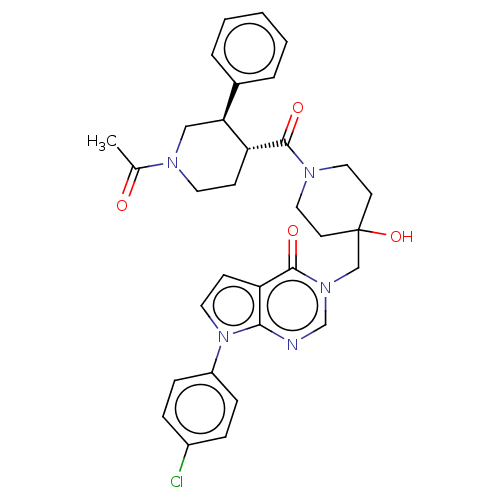
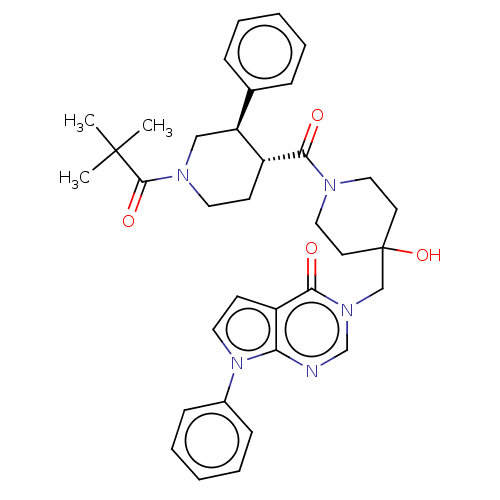
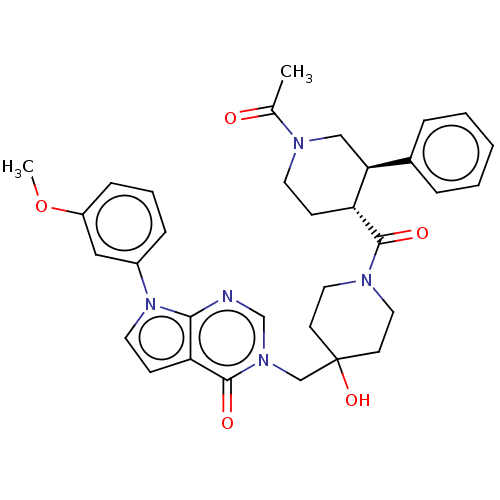
Affinity DataKi: 6.76nMAssay Description:Binding affinity to human mGluR5More data for this Ligand-Target Pair
Affinity DataKi: 36.3nMAssay Description:Binding affinity to human mGluR5More data for this Ligand-Target Pair
Affinity DataKi: 49.0nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 57.5nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 72.4nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 77.6nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 83.2nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 87.1nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 89.1nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 102nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 105nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 123nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 126nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 126nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 138nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 145nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 182nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 186nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 195nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 195nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 204nMAssay Description:Binding affinity to human mGluR5More data for this Ligand-Target Pair
Affinity DataKi: 229nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 316nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 457nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 741nMAssay Description:Displacement of [3H]-M-MPEP from mGluR5 in rat cerebrocortical membranesMore data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 19.6nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 28.7nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 31.6nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 37.7nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 39.3nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 41.2nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 48.6nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 48.6nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 49.9nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 52.4nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 52.9nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 56.7nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 61.5nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 64.8nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 68.7nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 70.2nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 74.2nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 74.8nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 83nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 88.4nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 88.5nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 89nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 96.1nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Les Laboratoires Servier
US Patent
Les Laboratoires Servier
US Patent
Affinity DataIC50: 102nMAssay Description:USP7 activity was measured using Rhodamine-110 c-terminal labelled Ubiquitin as a substrate (Viva Biosciences). Incubation with USP7 results in the r...More data for this Ligand-Target Pair