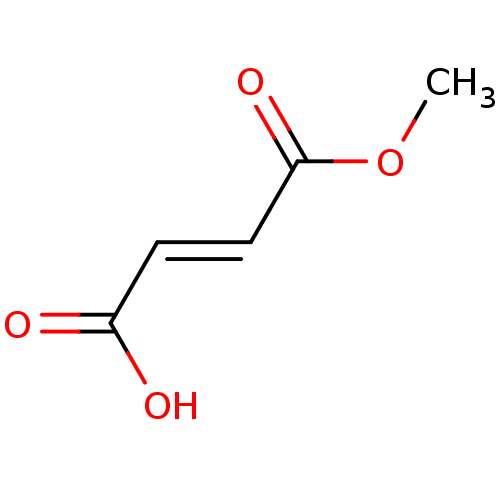
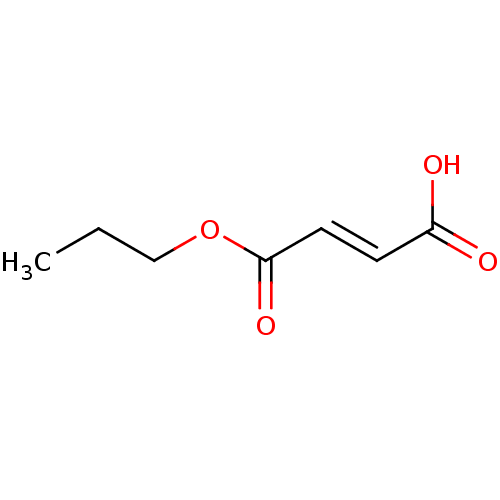
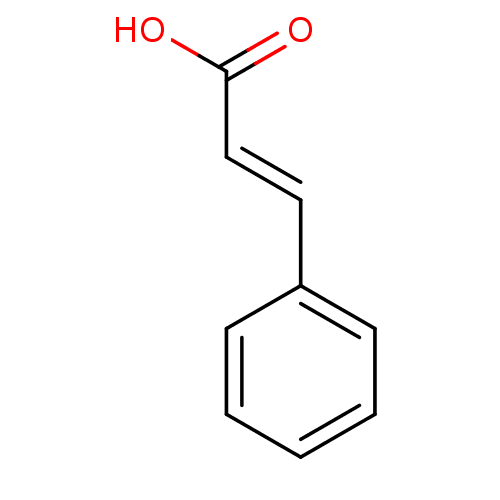
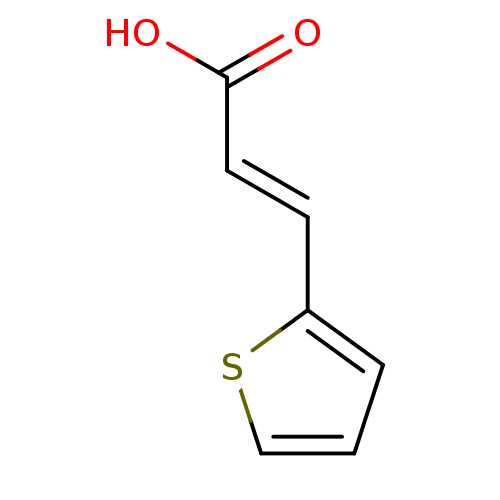
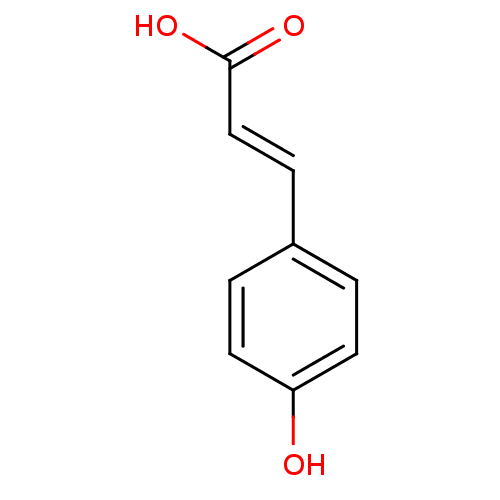
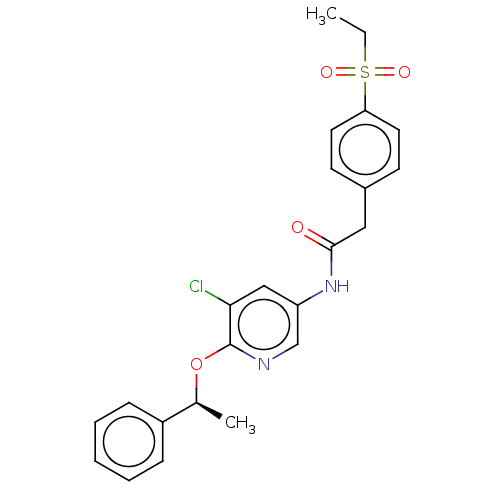
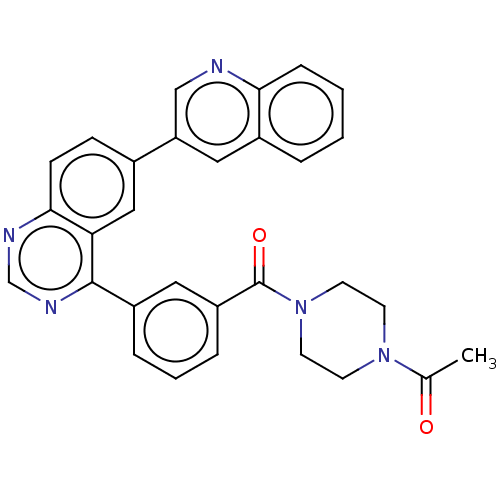
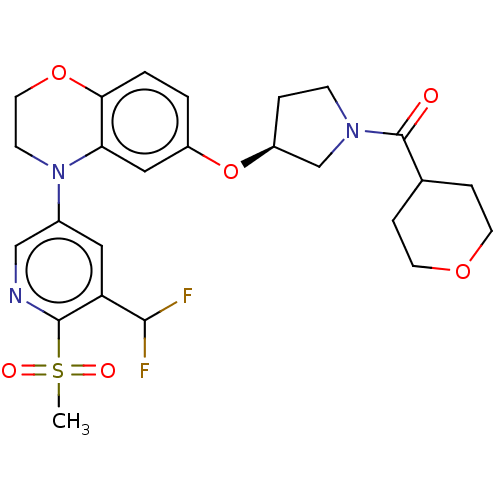
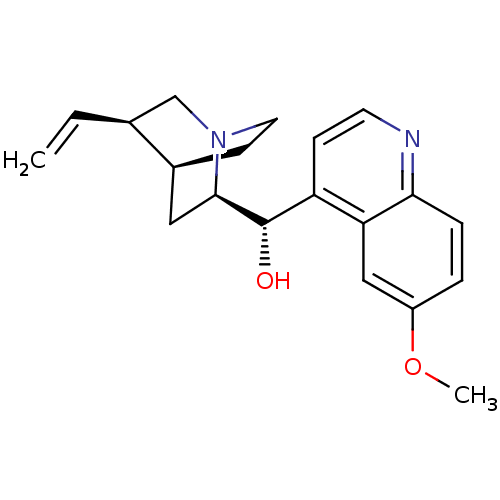
TargetEnterobactin synthase component E(Escherichia coli (strain K12))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
Affinity DataKi: 4.5nMAssay Description:Binding affinity to EntE corrected to substrate competitionMore data for this Ligand-Target Pair
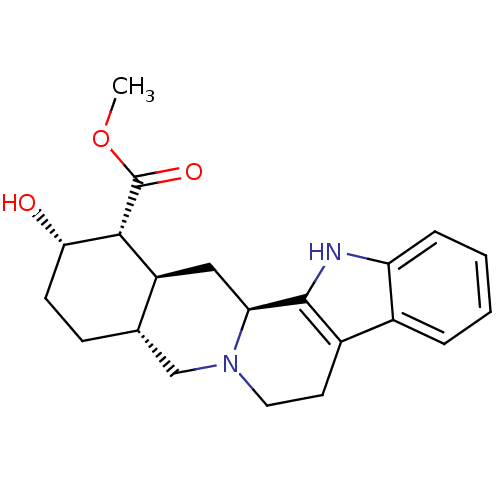
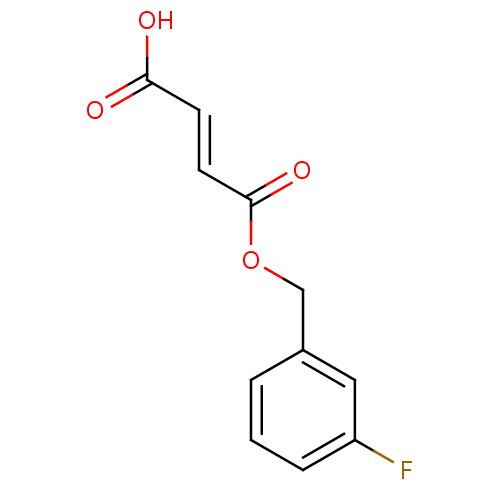
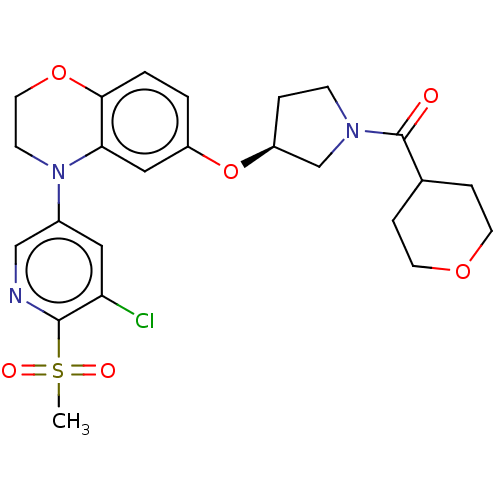
TargetEnterobactin synthase component E(Escherichia coli (strain K12))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
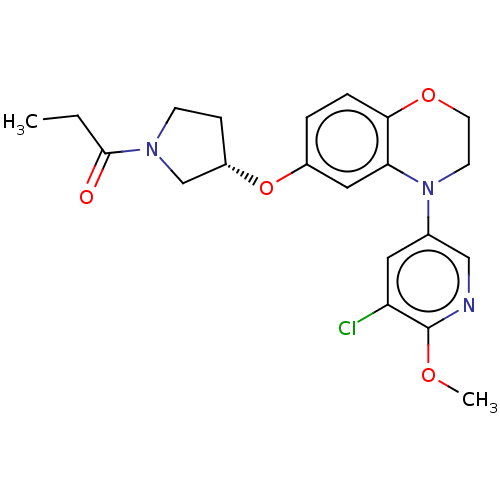
Affinity DataKi: 30nMAssay Description:Binding affinity for cytochrome P450 2D6More data for this Ligand-Target Pair
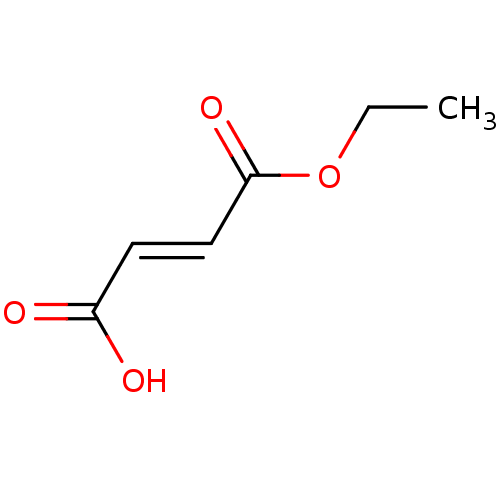
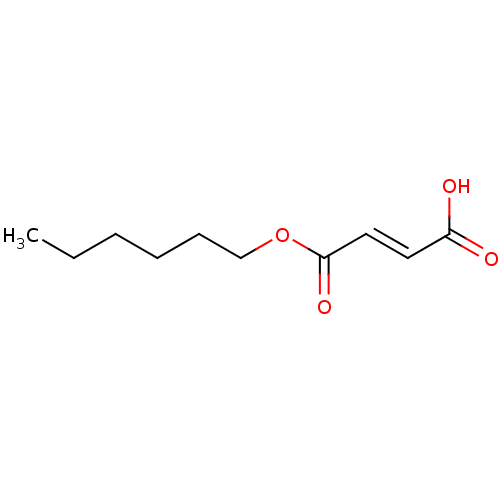
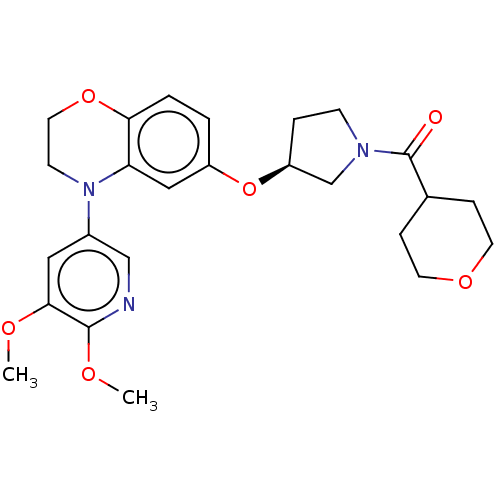
TargetEnterobactin synthase component E(Escherichia coli (strain K12))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Binding affinity to EntEMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Binding affinity for cytochrome P450 2D6More data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 760nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3.50E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.20E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.90E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 5.70E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+3nMAssay Description:Binding affinity for cytochrome P450 2D6More data for this Ligand-Target Pair
Affinity DataKi: 8.10E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.90E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 9.80E+3nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
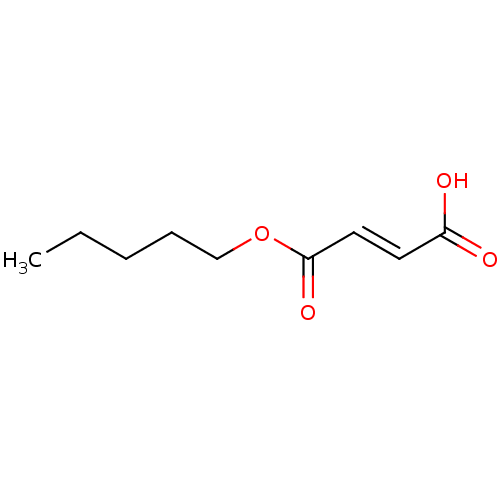
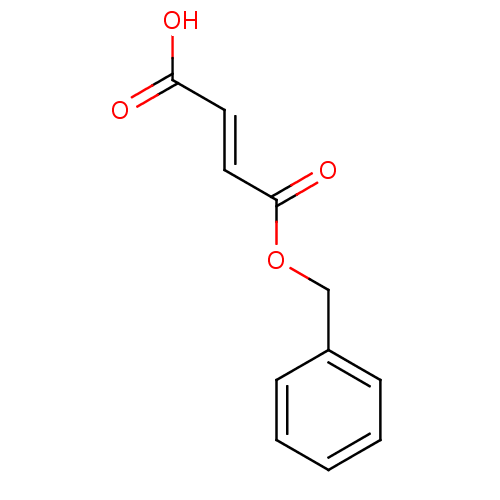
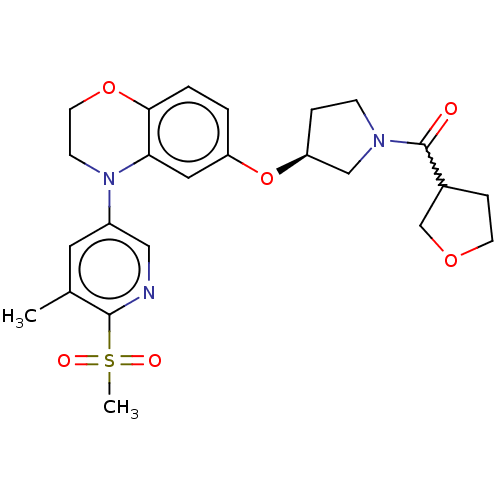
TargetEnterobactin synthase component E(Escherichia coli (strain K12))
University Of North Carolina
Curated by ChEMBL
University Of North Carolina
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Binding affinity to EntEMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in human HEK293T cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+4nMAssay Description:Binding affinity for cytochrome P450 2D6More data for this Ligand-Target Pair
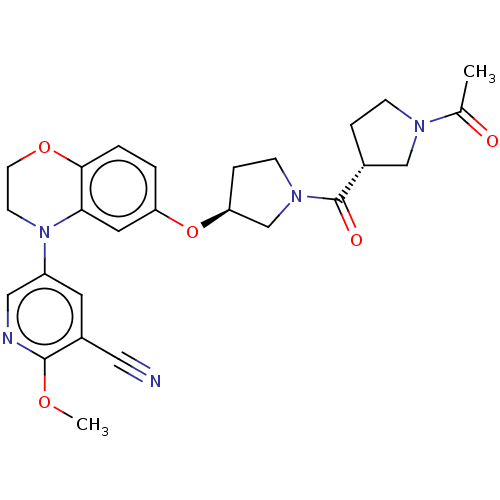
Affinity DataIC50: 0.120nMAssay Description:Inhibition of recombinant full length N-terminal His6-tagged LTA4H (unknown origin) expressed in Escherichia coli BL21 DE3 cells at enzyme concentrat...More data for this Ligand-Target Pair
TargetExtracellular calcium-sensing receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
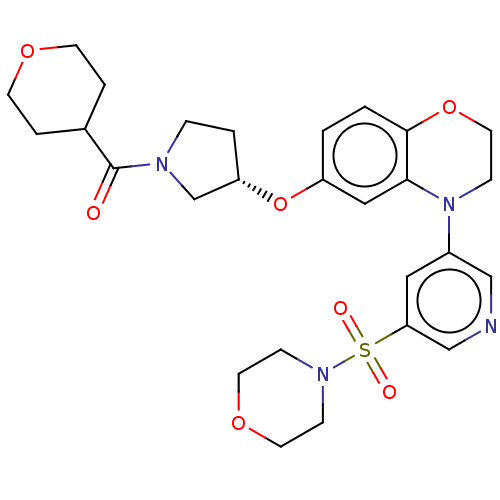
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inverse agonist activity at human His6-tagged RORgammat LBD (264 to 518 residues) assessed as reduction in biotinylated RIP140 co-activator recruitme...More data for this Ligand-Target Pair
TargetExtracellular calcium-sensing receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by...More data for this Ligand-Target Pair
TargetExtracellular calcium-sensing receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by...More data for this Ligand-Target Pair
TargetExtracellular calcium-sensing receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of recombinant full length N-terminal His6-tagged LTA4H (unknown origin) expressed in Escherichia coli BL21 DE3 cells at enzyme concentrat...More data for this Ligand-Target Pair
TargetExtracellular calcium-sensing receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by...More data for this Ligand-Target Pair
TargetExtracellular calcium-sensing receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by...More data for this Ligand-Target Pair
TargetExtracellular calcium-sensing receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant full length N-terminal His6-tagged LTA4H (unknown origin) expressed in Escherichia coli BL21 DE3 cells at enzyme concentrat...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)