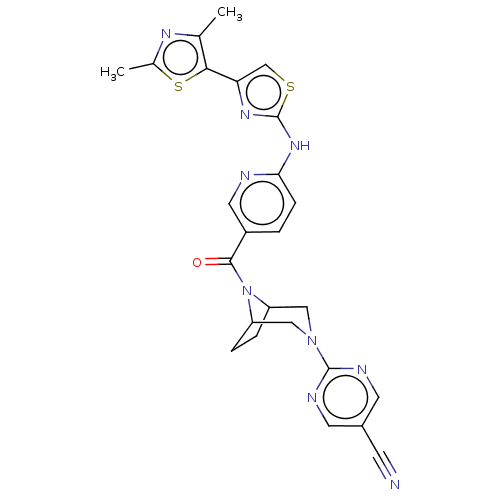
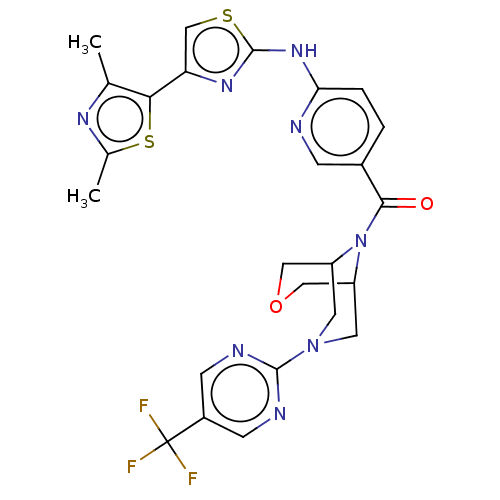
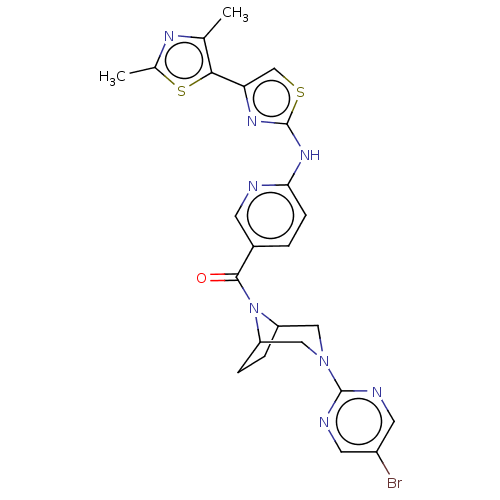
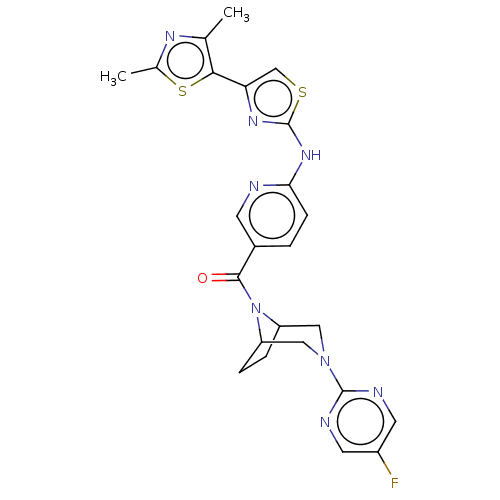
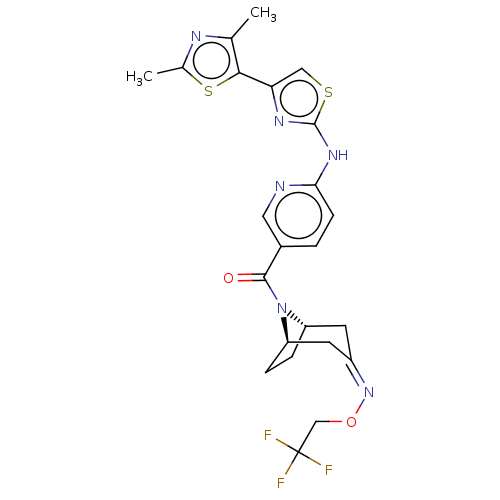
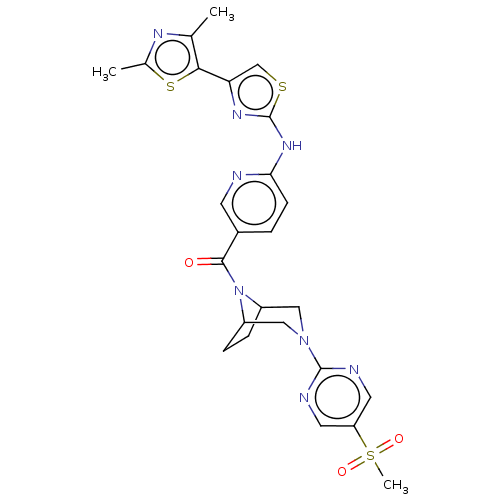
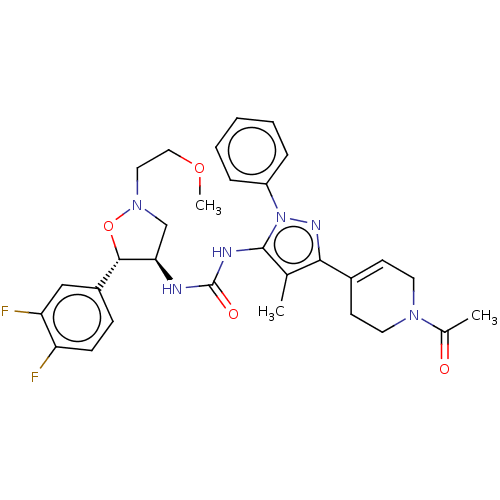
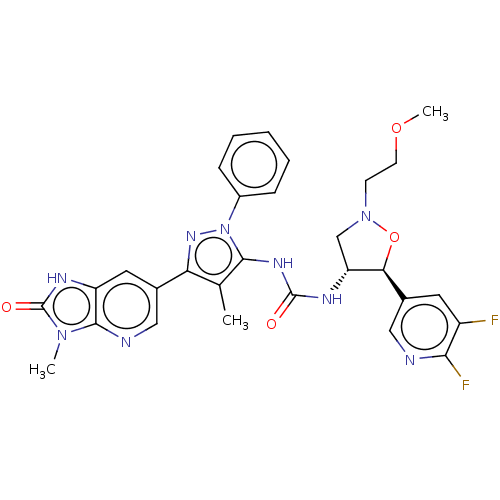
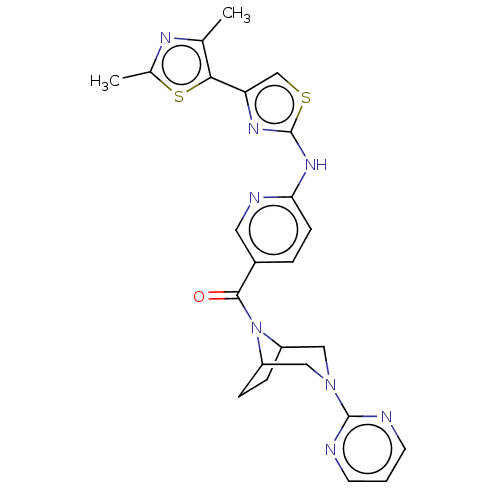
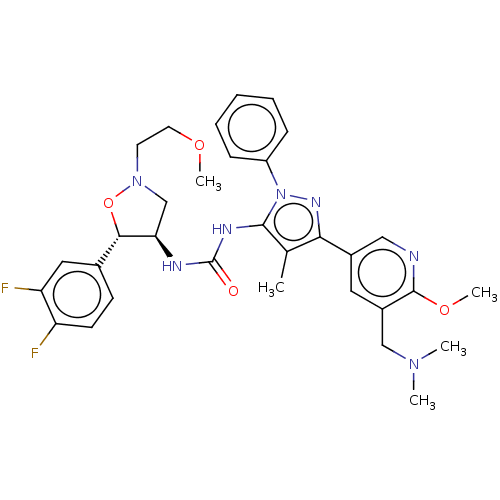
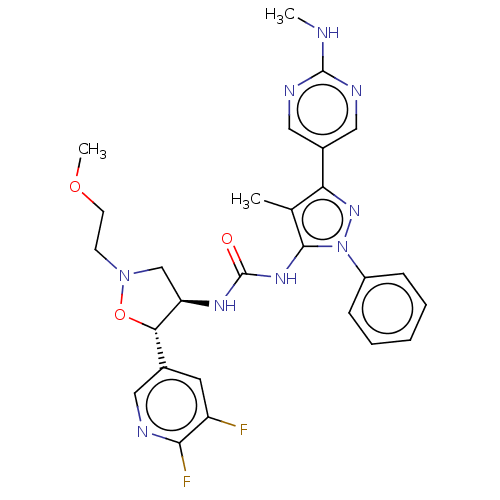
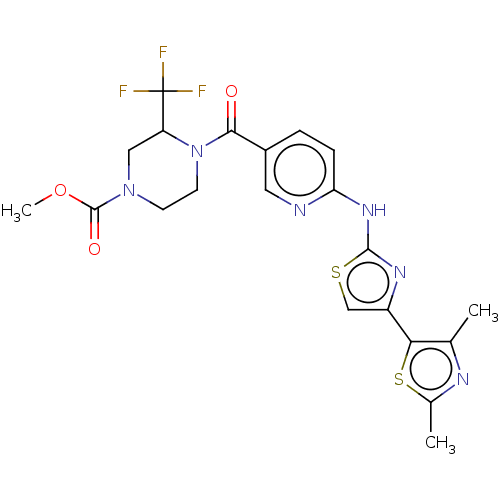
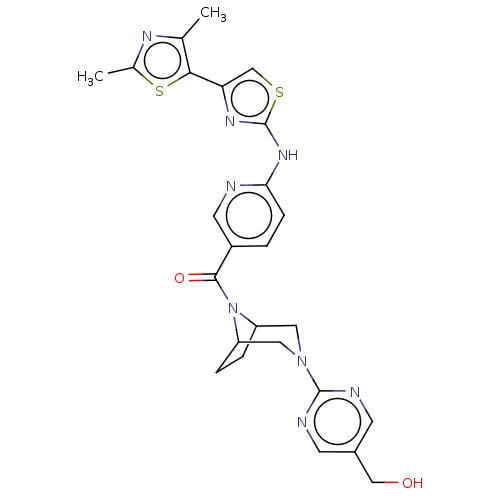
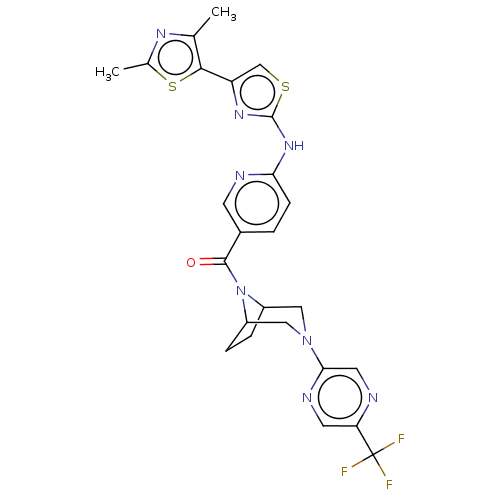
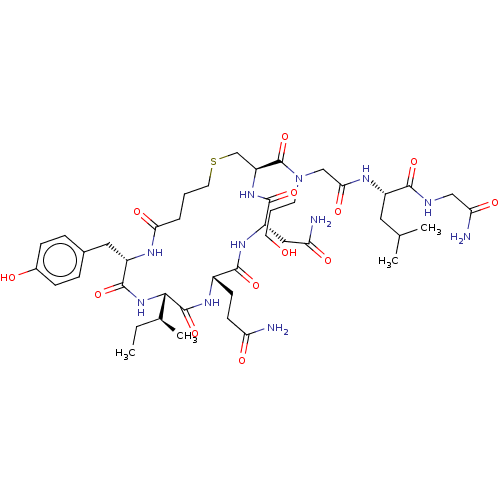
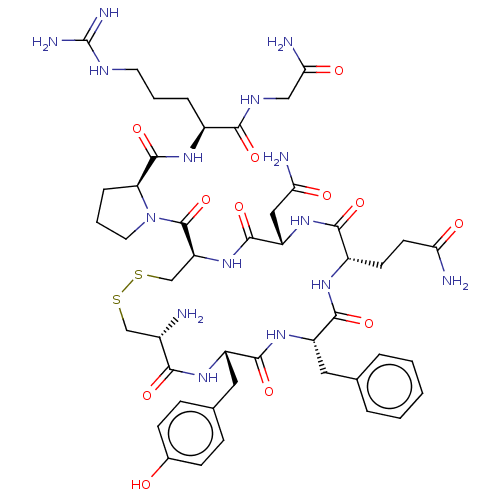
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.510nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.580nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Hamamatsu University School Of Medicine
Curated by ChEMBL
Hamamatsu University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]-(E)-N1-(2-methoxybenzyl)cinnamamidine from human NR1a/NR2b receptor expressed in mouse Ltk cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Hamamatsu University School Of Medicine
Curated by ChEMBL
Hamamatsu University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 3.09nMAssay Description:Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
Affinity DataKi: 4.40nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Hamamatsu University School Of Medicine
Curated by ChEMBL
Hamamatsu University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 5.75nMAssay Description:Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 6.60nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Hamamatsu University School Of Medicine
Curated by ChEMBL
Hamamatsu University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 7.28nMAssay Description:Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Hamamatsu University School Of Medicine
Curated by ChEMBL
Hamamatsu University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranesMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Hamamatsu University School Of Medicine
Curated by ChEMBL
Hamamatsu University School Of Medicine
Curated by ChEMBL
Affinity DataKi: 32.5nMAssay Description:Displacement of [3H]ifenprodil from NR2B receptor in rat cortical synaptic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
Affinity DataKi: 134nMAssay Description:Affinity for the glycine binding site of the NMDA receptor using [3H]- 5,7- dichloro -kynurenic acid as radio-ligandMore data for this Ligand-Target Pair
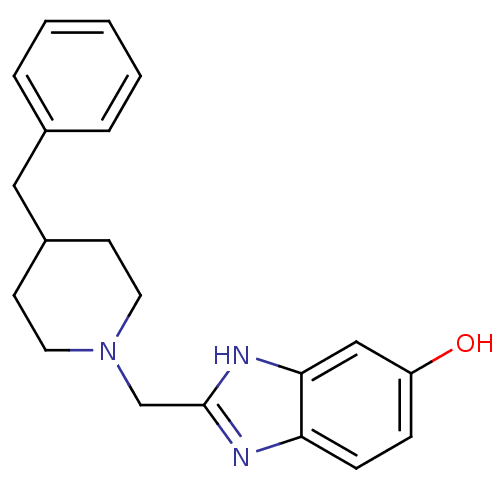
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.180nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.220nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.220nMAssay Description:Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.290nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.310nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.360nMAssay Description:Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.390nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity at human TRPV4 assessed as inhibition of hypotonicity-induced activationMore data for this Ligand-Target Pair
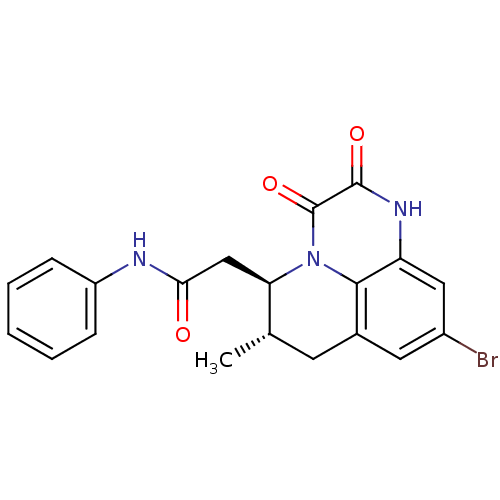
Affinity DataIC50: 0.480nMAssay Description:Seven point five μL per well of human TrkA (PV3144, Lifetechnologies, final concentration: 1 nmol/L) suspended in the assay buffer (100 mmol/L 4...More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Seven point five μL per well of human TrkA (PV3144, Lifetechnologies, final concentration: 1 nmol/L) suspended in the assay buffer (100 mmol/L 4...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.520nMAssay Description:Seven point five μL per well of human TrkA (PV3144, Lifetechnologies, final concentration: 1 nmol/L) suspended in the assay buffer (100 mmol/L 4...More data for this Ligand-Target Pair
Affinity DataIC50: 0.520nMAssay Description:Seven point five μL per well of human TrkA (PV3144, Lifetechnologies, final concentration: 1 nmol/L) suspended in the assay buffer (100 mmol/L 4...More data for this Ligand-Target Pair
Affinity DataIC50: 0.520nMAssay Description:Seven point five μL per well of human TrkA (PV3144, Lifetechnologies, final concentration: 1 nmol/L) suspended in the assay buffer (100 mmol/L 4...More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:Seven point five μL per well of human TrkA (PV3144, Lifetechnologies, final concentration: 1 nmol/L) suspended in the assay buffer (100 mmol/L 4...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.570nMAssay Description:Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Seven point five μL per well of human TrkA (PV3144, Lifetechnologies, final concentration: 1 nmol/L) suspended in the assay buffer (100 mmol/L 4...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.650nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
Shionogi
Curated by ChEMBL
Shionogi
Curated by ChEMBL
Affinity DataIC50: 0.710nMAssay Description:Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi...More data for this Ligand-Target Pair



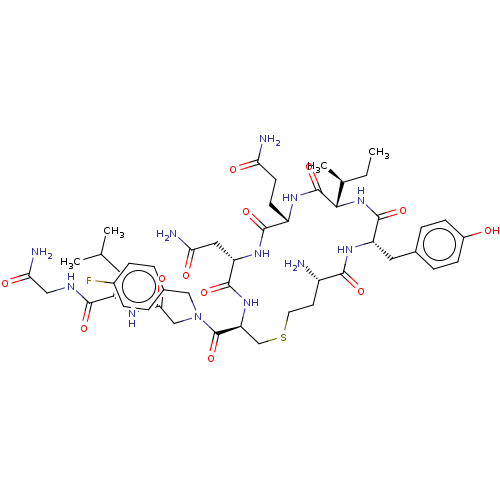

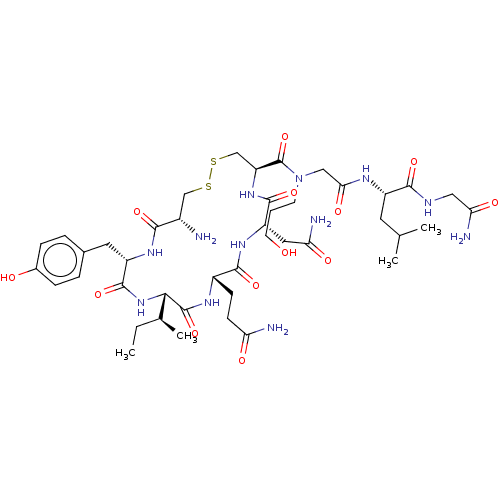










 3D Structure (crystal)
3D Structure (crystal)