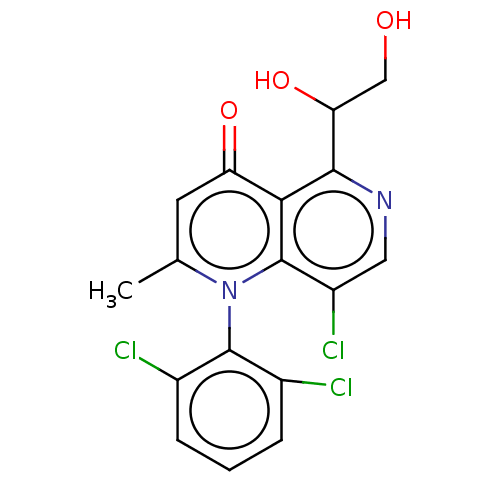
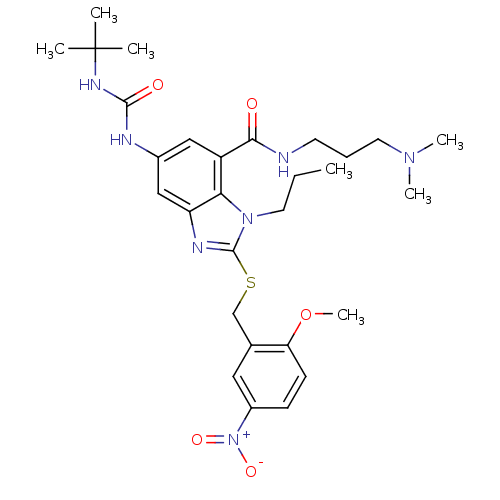
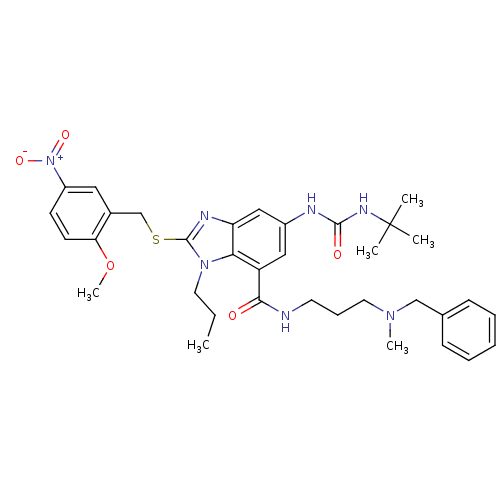
TargetSerine/threonine-protein kinase WNK1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
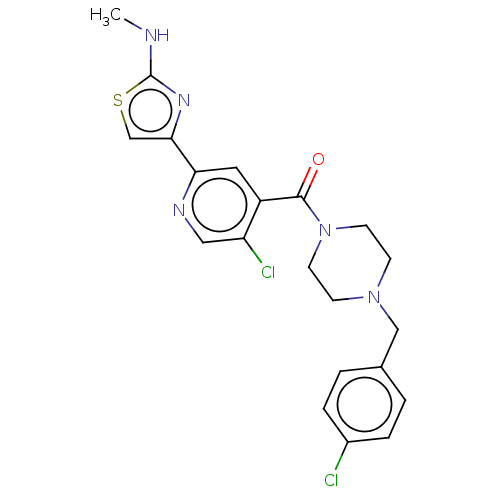
Affinity DataKi: 32nMAssay Description:Allosteric inhibition of WNK1 (unknown origin) expressed in HEK293 cells co-expressing flag-OSR1 assessed as reduction in sorbitol-stimulated OSR1 ph...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.3 T: 2°CAssay Description:Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin
Curated by ChEMBL
Bayer Yakuhin
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin
Curated by ChEMBL
Bayer Yakuhin
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin
Curated by ChEMBL
Bayer Yakuhin
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibitory concentration of the compound towards rat leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Allosteric inhibition of recombinant human N-terminal GST-tagged WNK1 catalytic domain (1 to 491 residues) expressed in baculovirus expression system...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibitory concentration of the compound towards human leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.3 T: 2°CAssay Description:Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Allosteric inhibition of recombinant human N-terminal GST-tagged WNK1 catalytic domain (1 to 491 residues) expressed in baculovirus expression system...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 6nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.3 T: 2°CAssay Description:Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 8nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 9nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 9nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.3 T: 2°CAssay Description:Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin
Curated by ChEMBL
Bayer Yakuhin
Curated by ChEMBL
Affinity DataIC50: 9.10nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 11nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory concentration of the compound towards rat leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 13nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 13nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 14nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibitory concentration of the compound towards rat leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 15nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 15nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 16nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin
Curated by ChEMBL
Bayer Yakuhin
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 17nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin
Curated by ChEMBL
Bayer Yakuhin
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence methodMore data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibitory concentration against human leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 19nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibitory concentration against rat leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory concentration of the compound towards human leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 21nMpH: 7.4 T: 2°CAssay Description:CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibitory concentration against human leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibitory concentration against rat leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration of the compound towards human leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 23nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 23nMpH: 7.4 T: 2°CAssay Description:CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory concentration against human leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory concentration against rat leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 24nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 25nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 25nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #...More data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 26nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibitory concentration against human leutinizing releasing hormone receptorMore data for this Ligand-Target Pair
TargetG protein-activated inward rectifier potassium channel 1/4(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 28nMAssay Description:Quattro is controlled using IonWorks v2 software to perform the following steps:a. Add 3.5 ul cells plus 3.5 ul external buffer to wells of Quattro P...More data for this Ligand-Target Pair










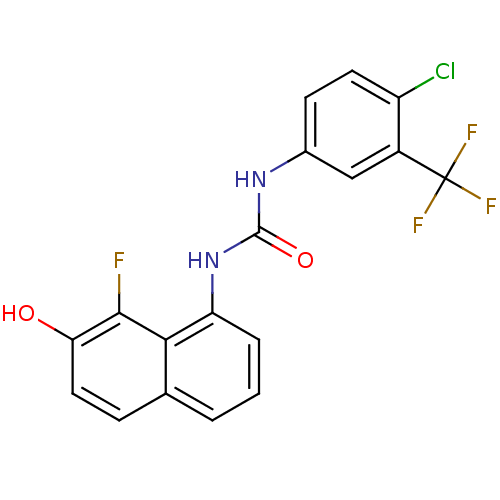




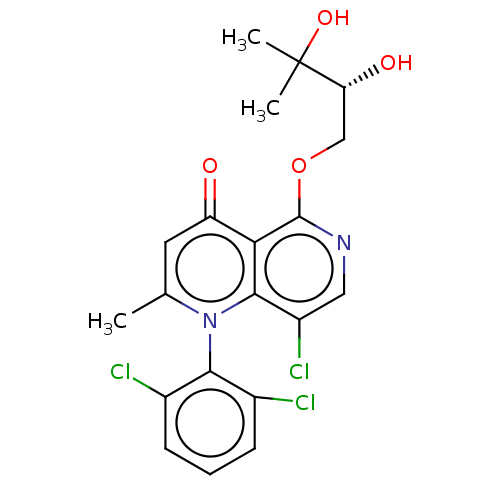




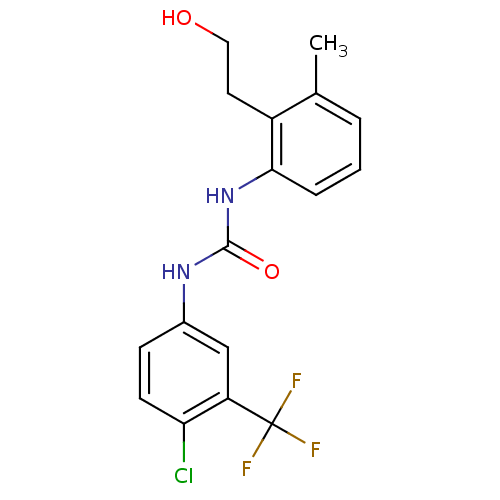


 3D Structure (crystal)
3D Structure (crystal)