Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB (change energy unit to kcal/mol)
 Found 33 hits in this display
Found 33 hits in this display
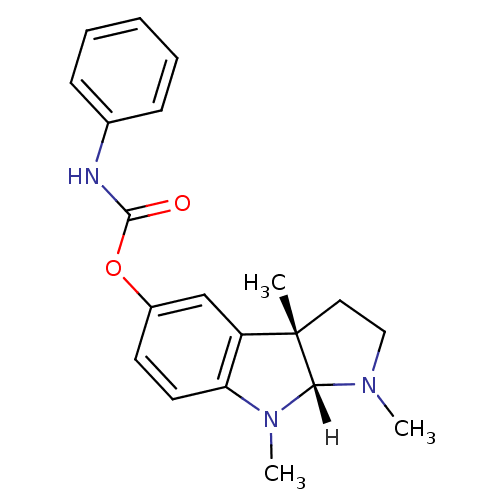 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)IC50: 28.3nMAssay Description:Inhibition of AChE in Wistar rat brain homogenate using acetylthiocholine iodide as substrate after 0.5 hrs by Ellman's methodMore data for this Ligand-Target Pair
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
Cholinesterase(Homo sapiens (Human))University Of North Carolina At Chapel Hill
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
 BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)
BDBM10622((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)IC50: 3.00E+4nMAssay Description:Inhibition of BuChE in Wistar rat plasma using acetylthiocholine iodide as substrate after 0.5 hrs by Ellman's methodMore data for this Ligand-Target Pair
 BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)
BDBM10958((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)