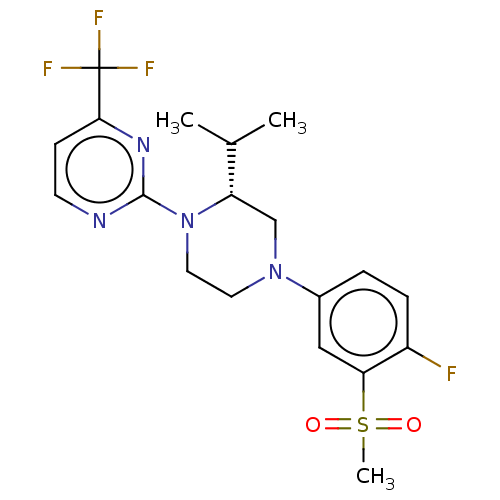
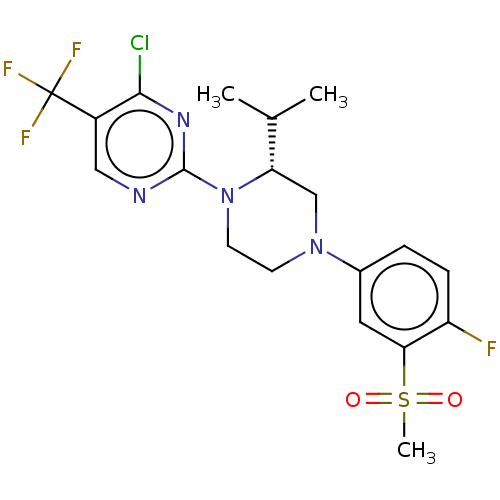
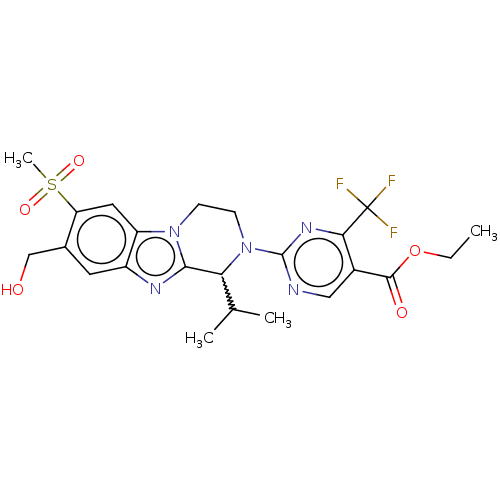
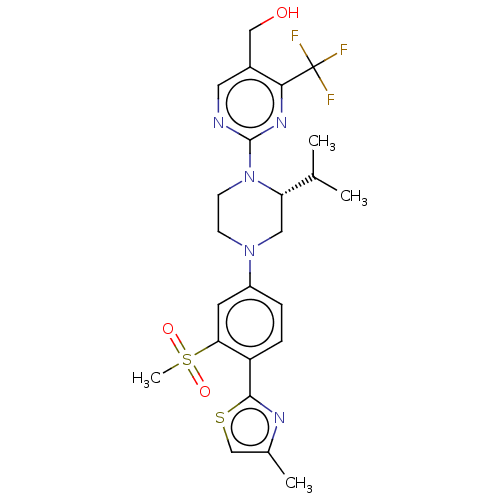
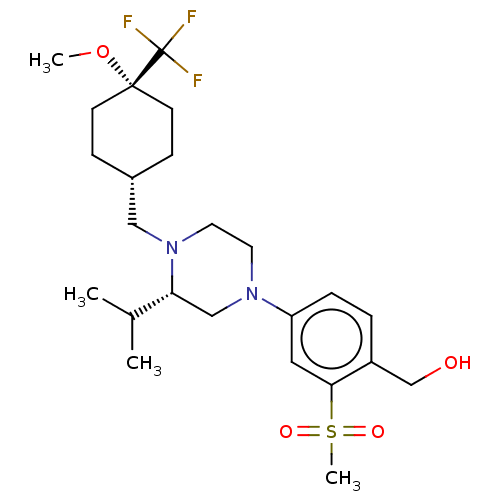
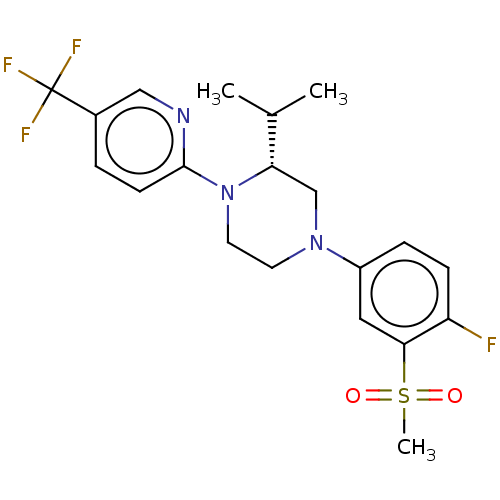
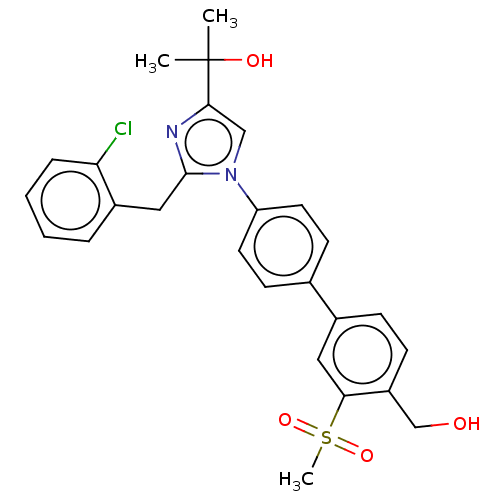
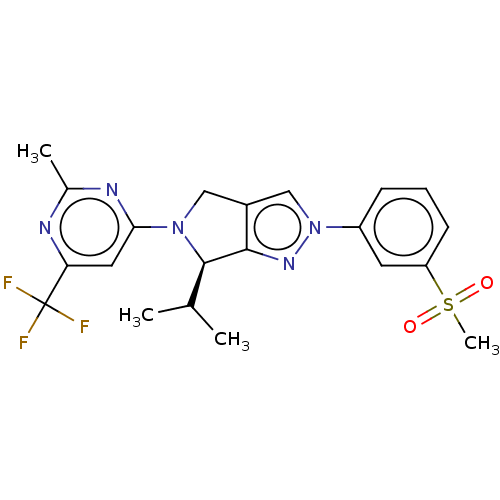
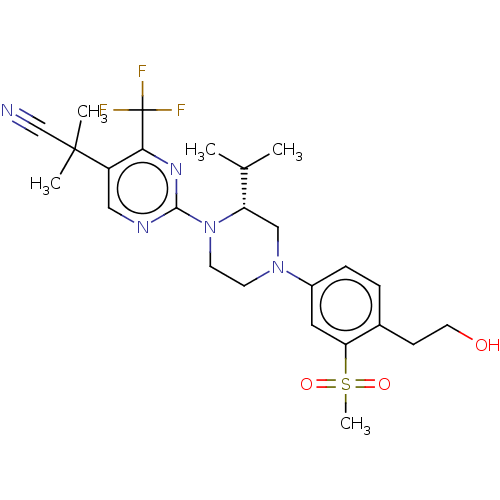
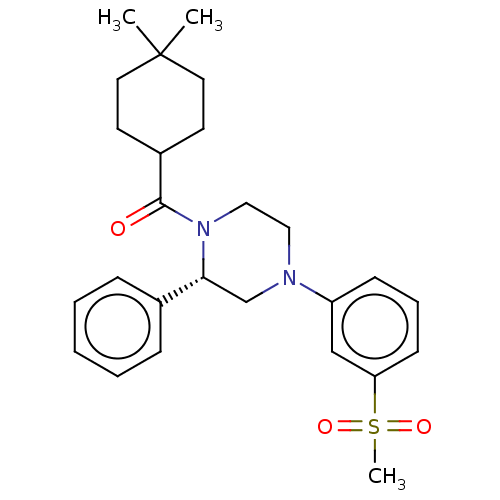
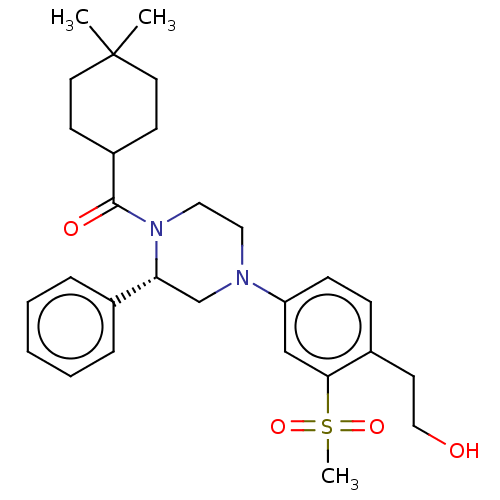
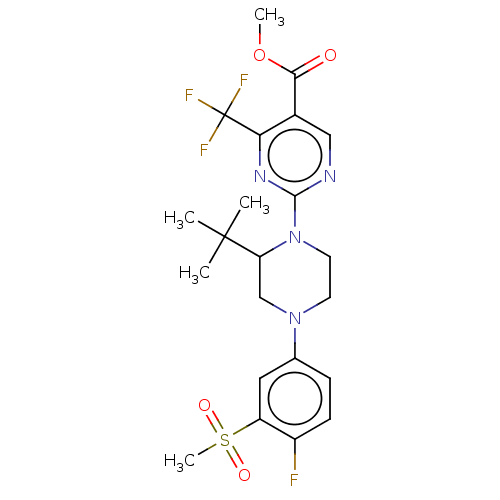
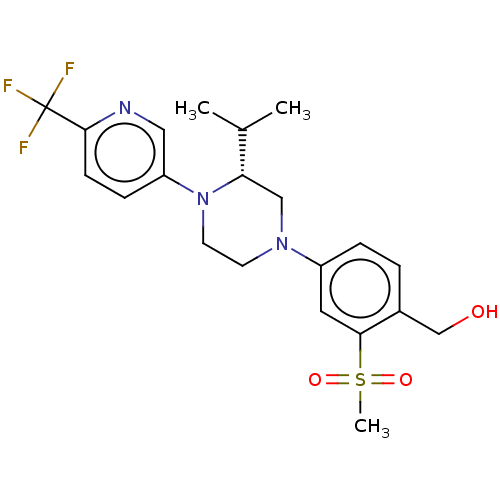
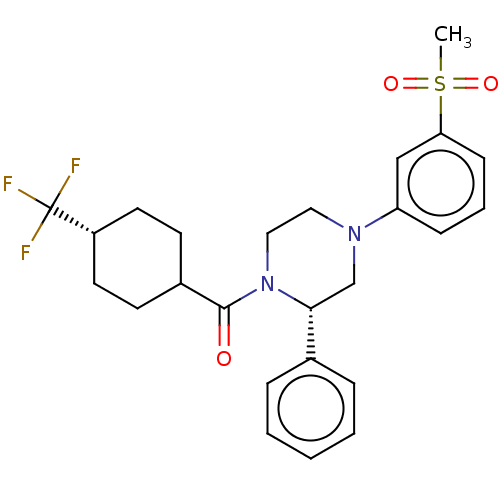
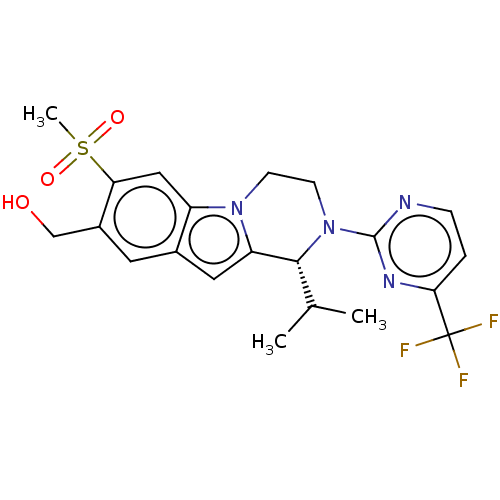
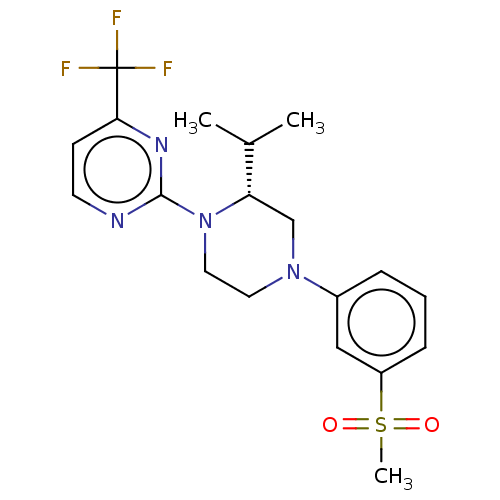
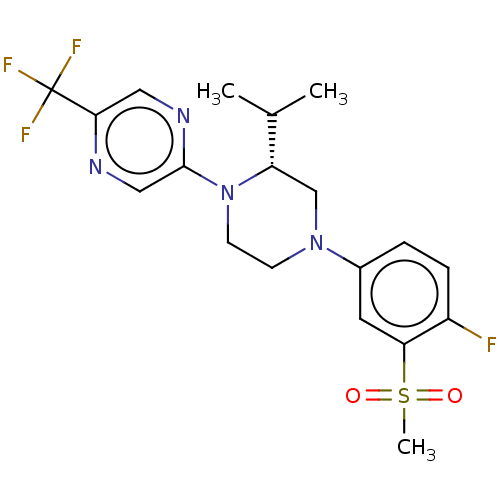
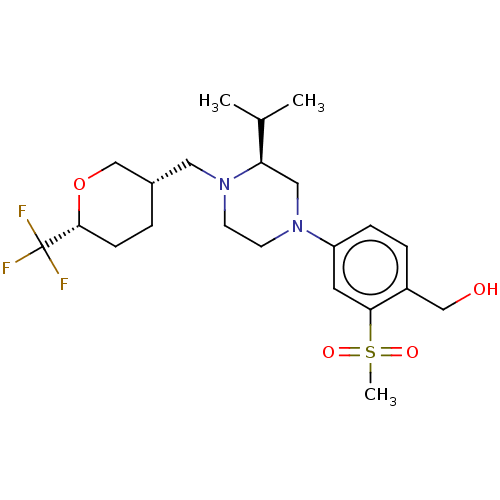
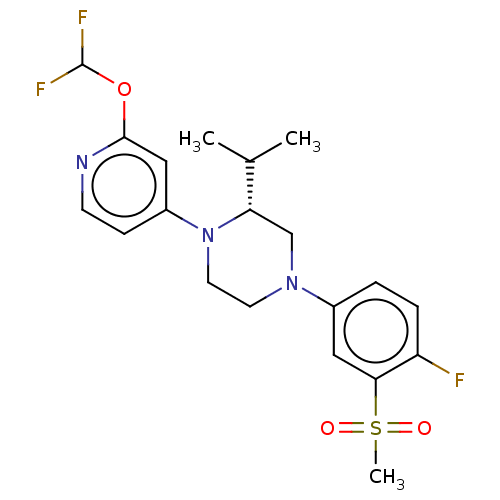
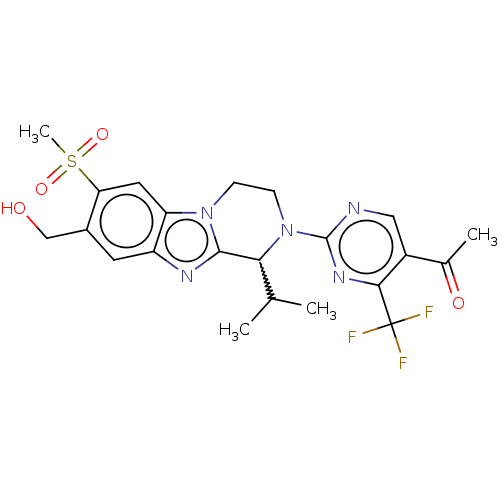
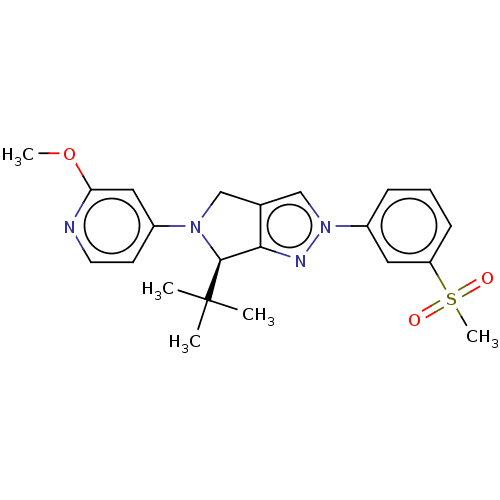
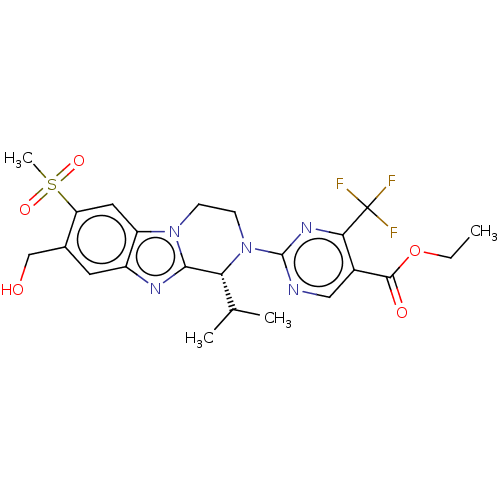
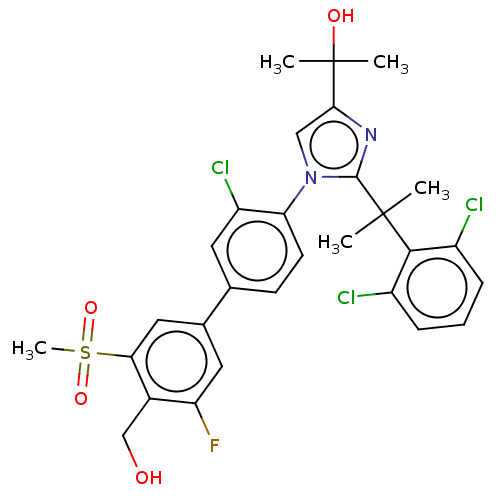
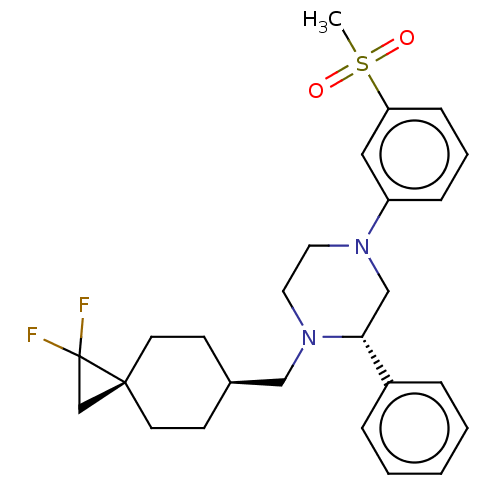
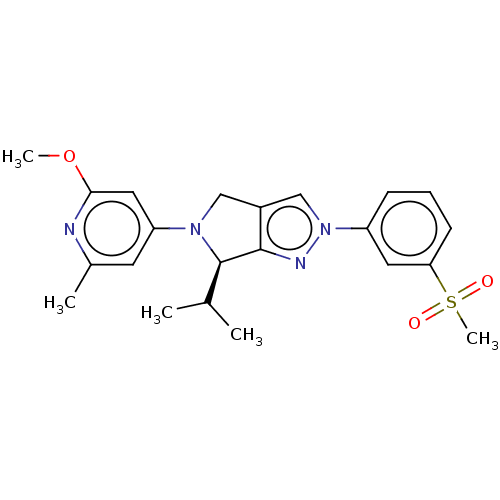
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
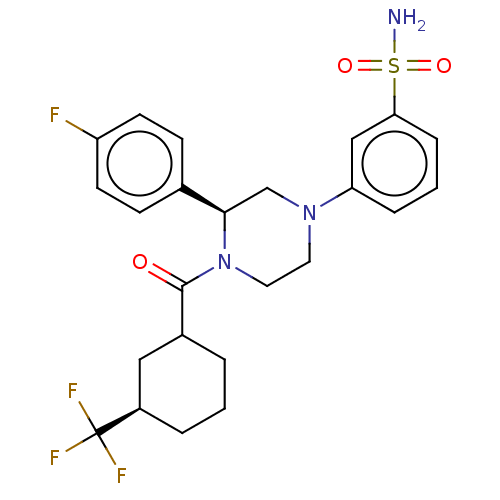
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
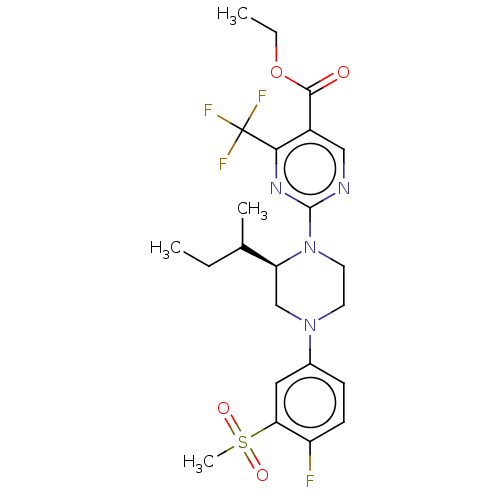
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
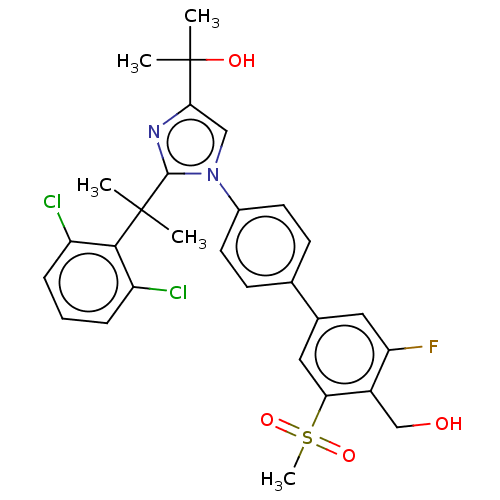
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 23nM ΔG°: -43.6kJ/moleT: 2°CAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -43.3kJ/moleT: 2°CAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy...More data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy...More data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 42nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 44nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 46nM ΔG°: -41.9kJ/moleT: 2°CAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 49nM ΔG°: -41.7kJ/moleT: 2°CAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy...More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair

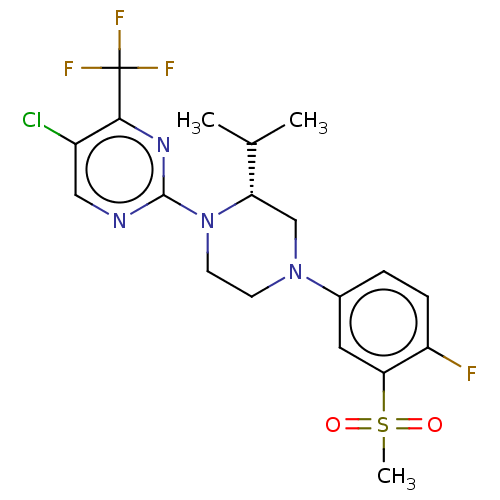
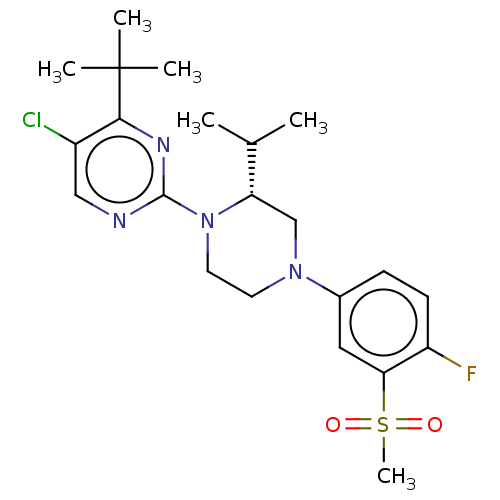
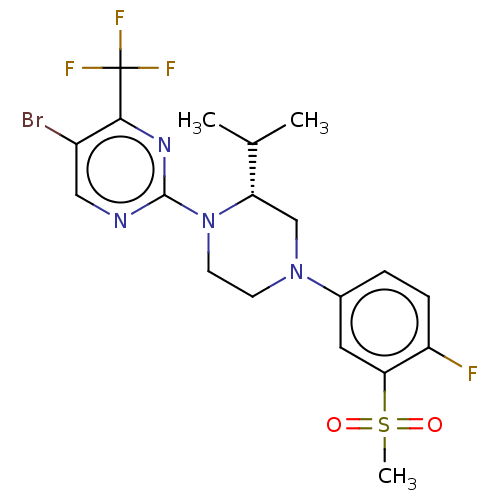
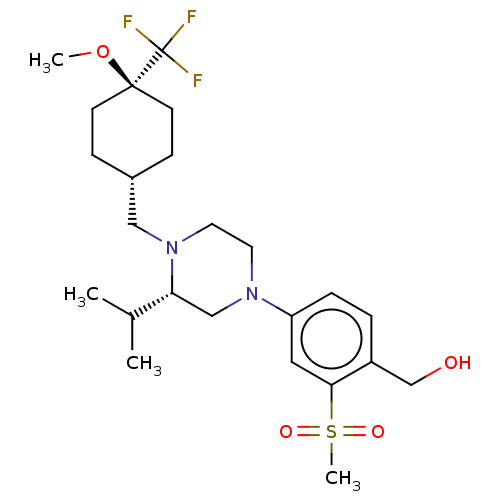
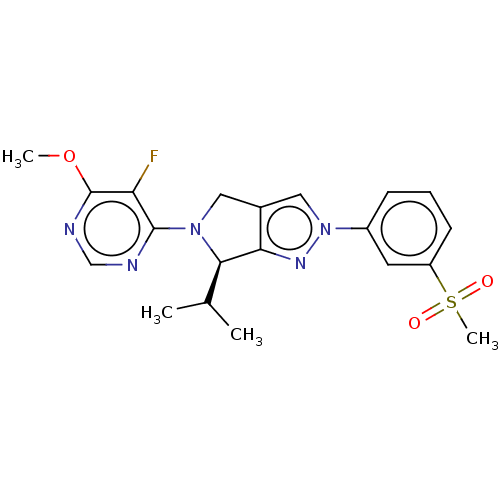
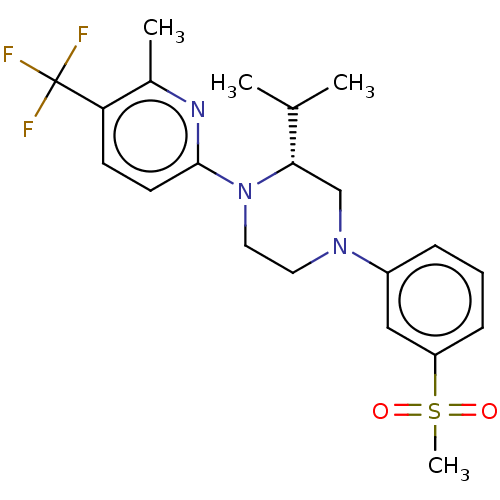
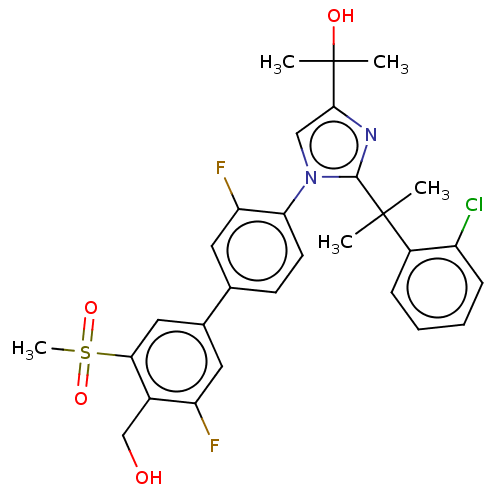
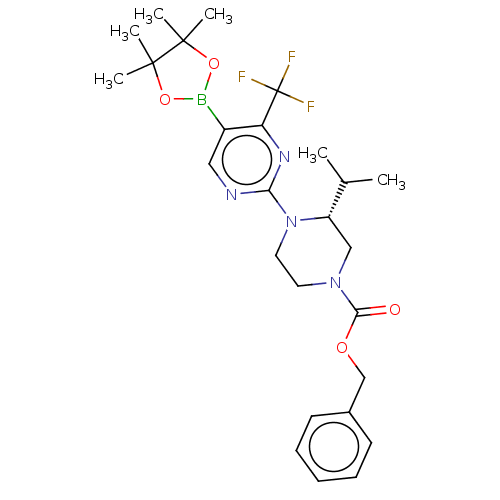
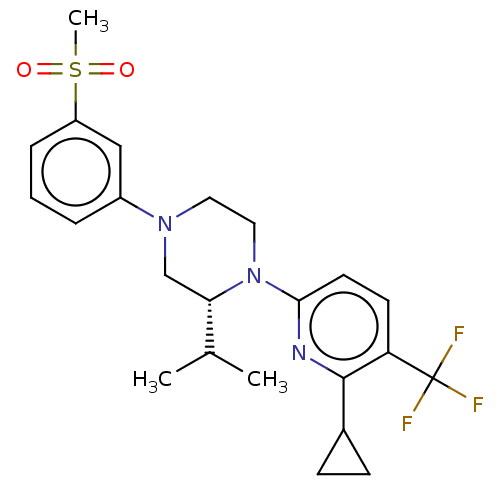
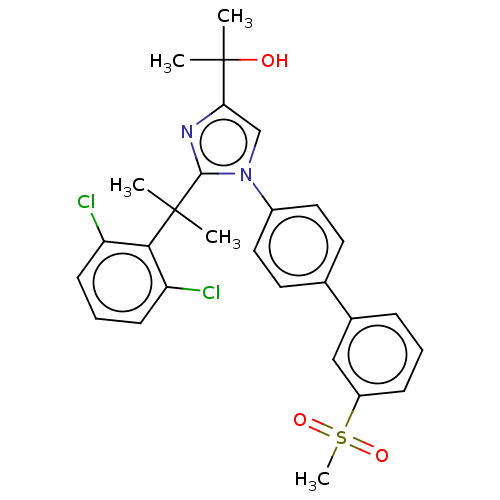
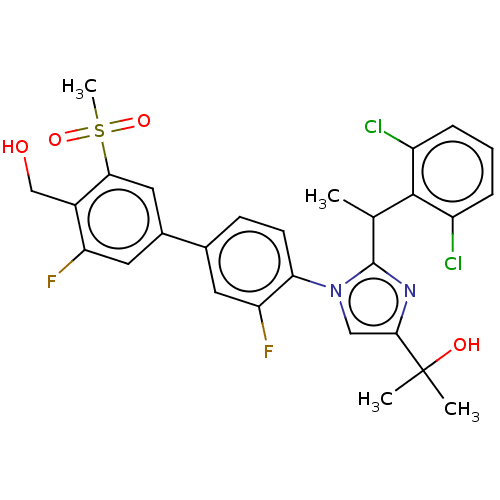
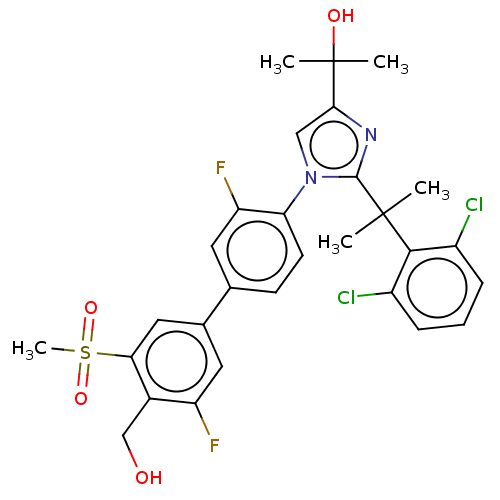
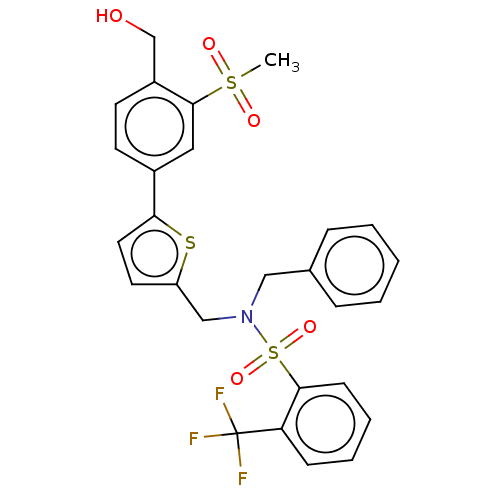
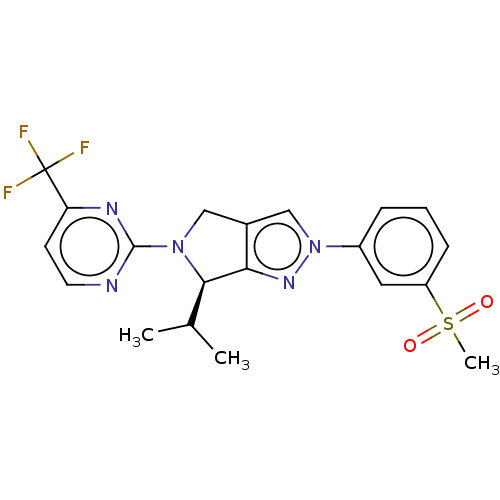
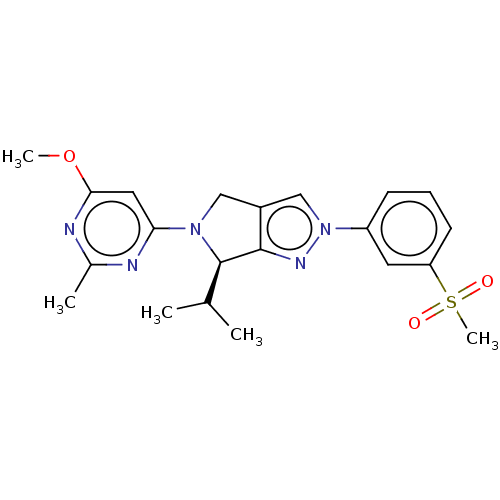
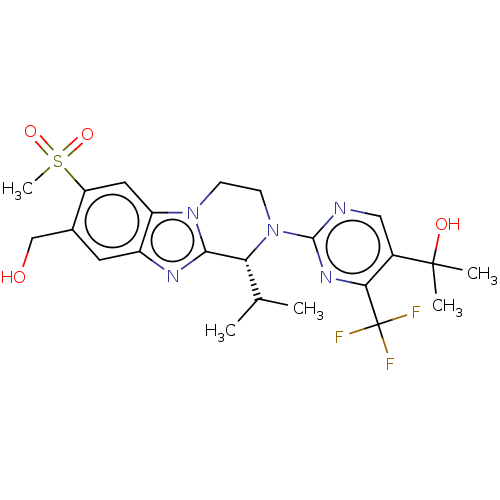
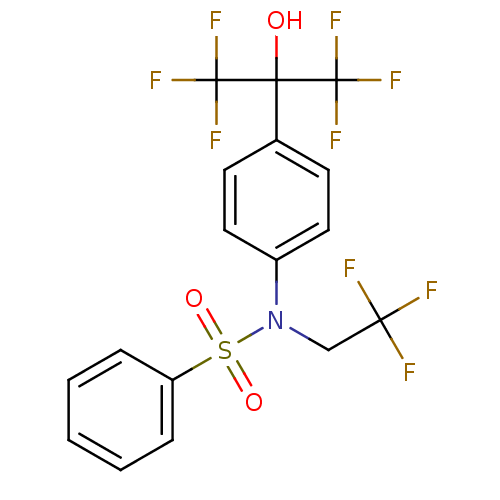
 3D Structure (crystal)
3D Structure (crystal)