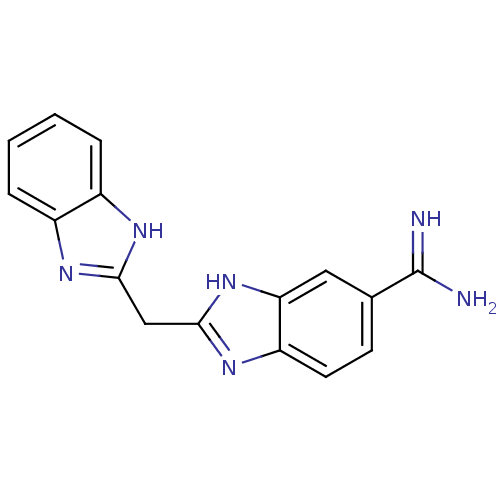
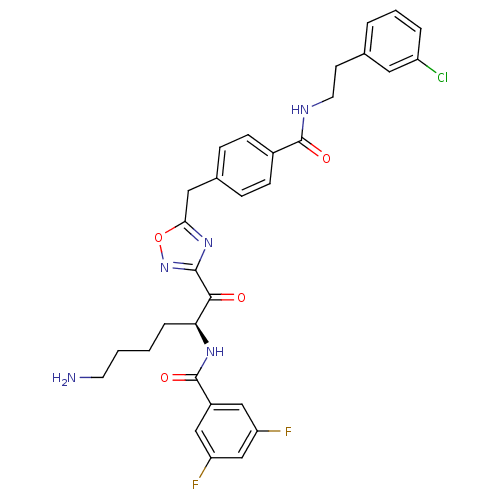
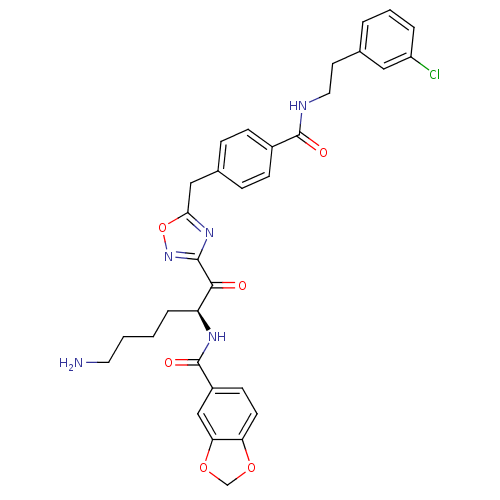
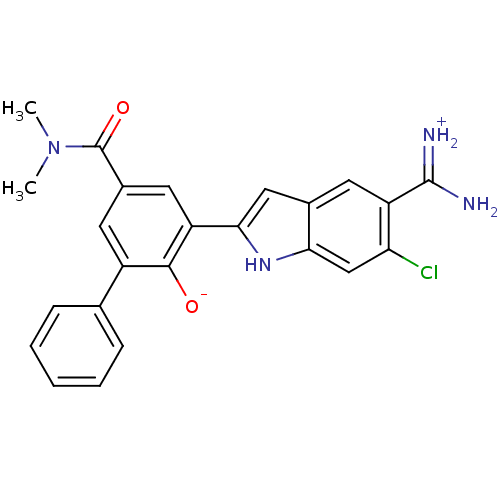
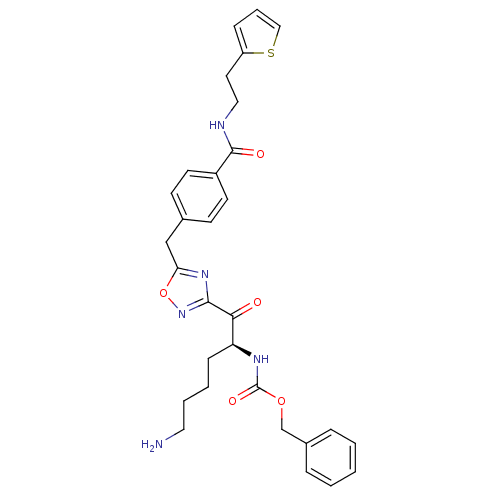
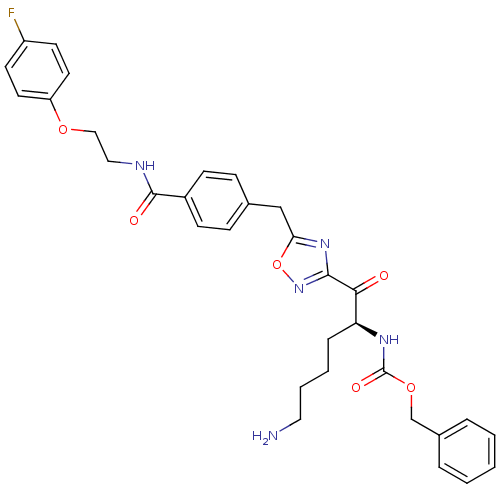
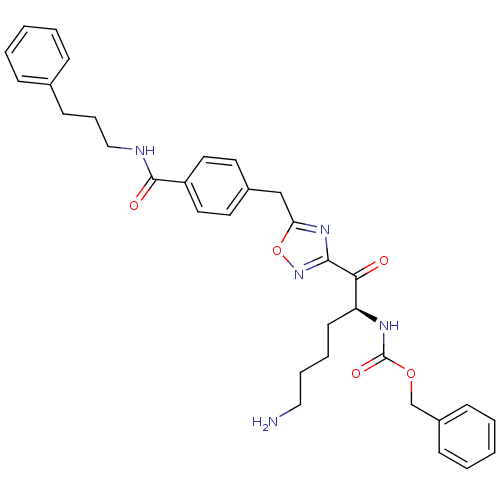
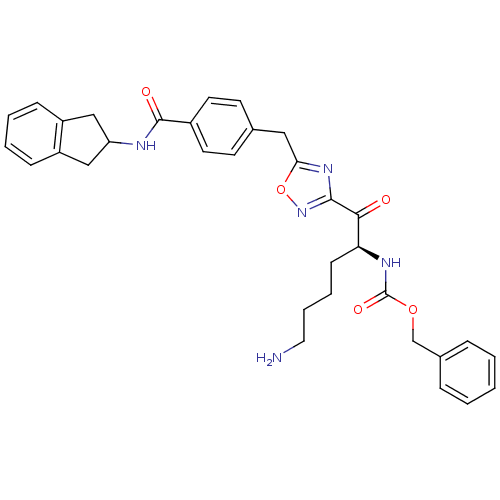
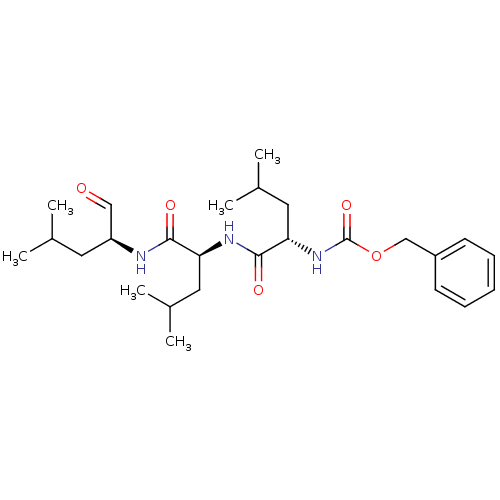
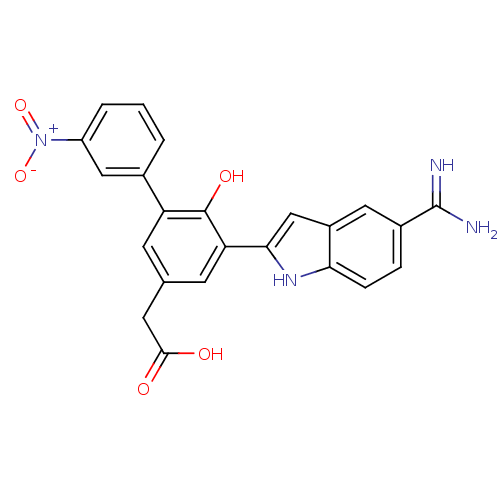
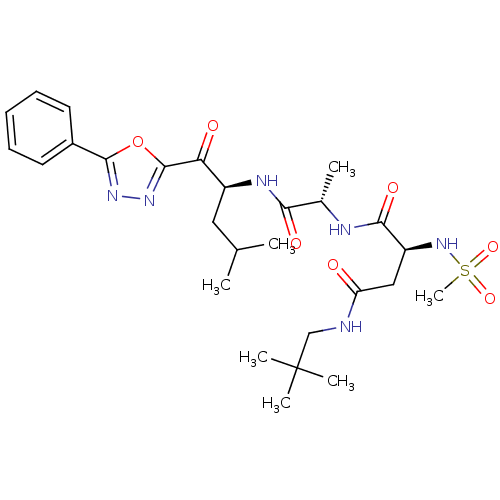
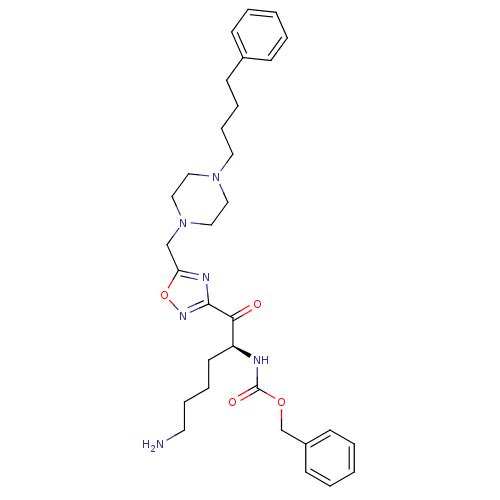
Affinity DataKi: 0.5nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.720nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 0.720nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity to thrombin in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -11.3kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity against cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -11.3kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 5.40nM ΔG°: -11.2kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibition of chymotrypsin-like proteasome activity of human 20S proteasomeMore data for this Ligand-Target Pair
Affinity DataKi: 5.80nMAssay Description:Inhibition of human beta tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to FXa in presence of Zn2+More data for this Ligand-Target Pair
Affinity DataKi: 6nM ΔG°: -11.1kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair


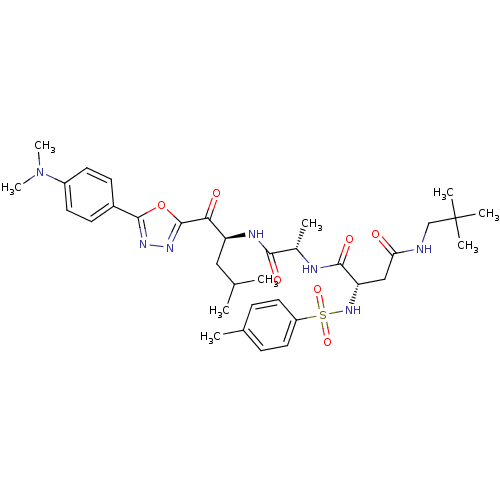
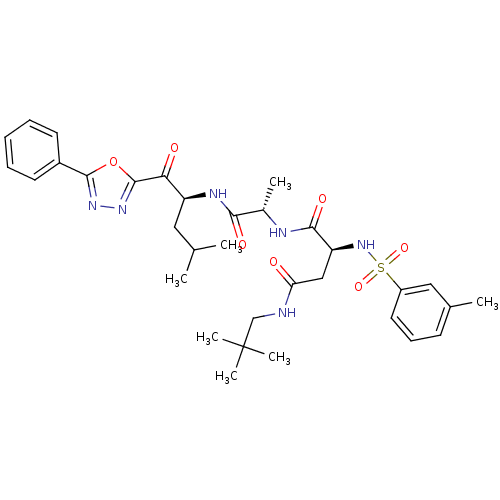
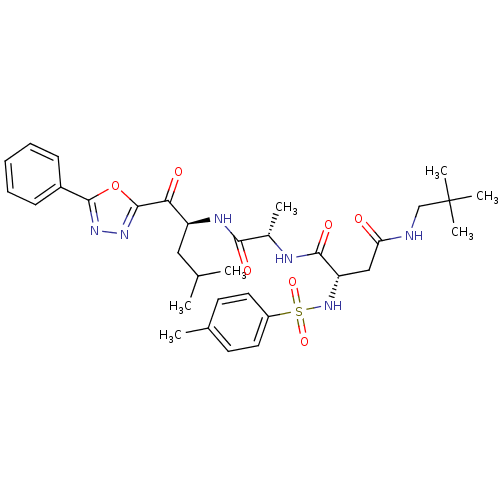
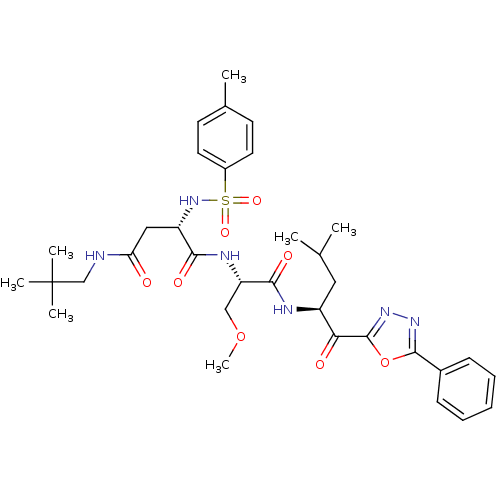

 3D Structure (crystal)
3D Structure (crystal)