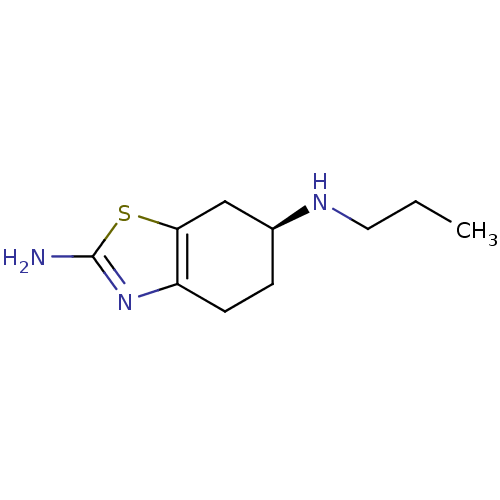
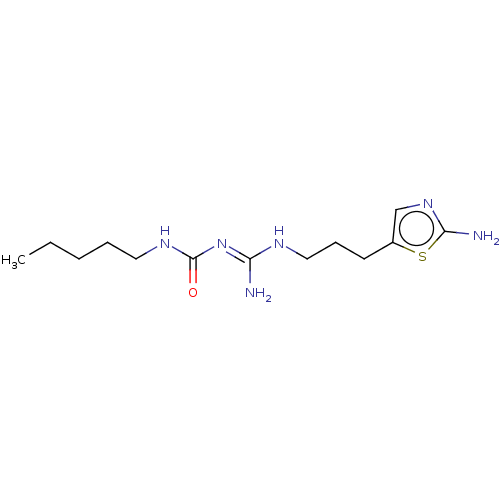
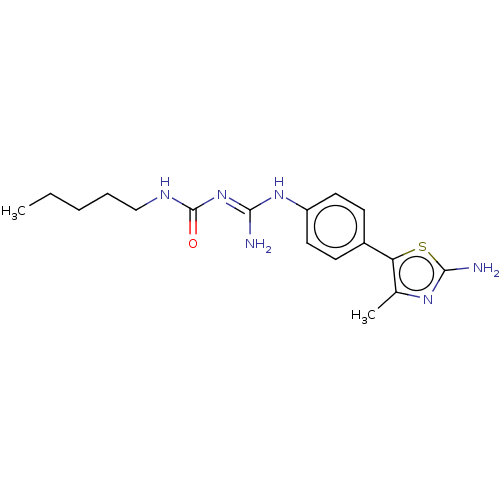
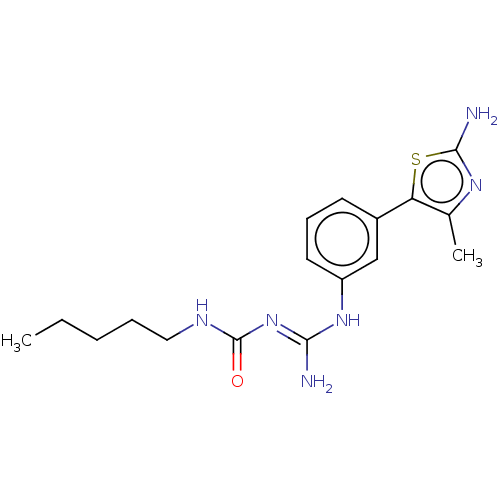
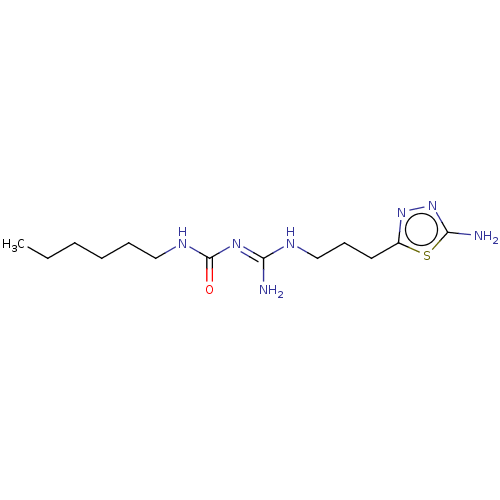
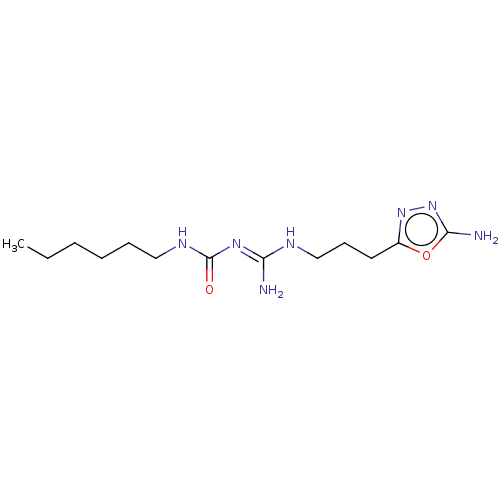
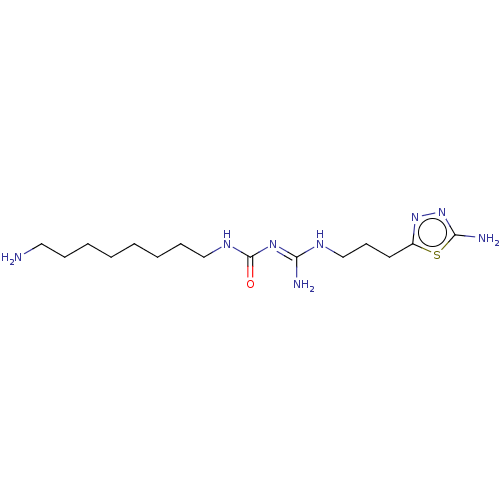
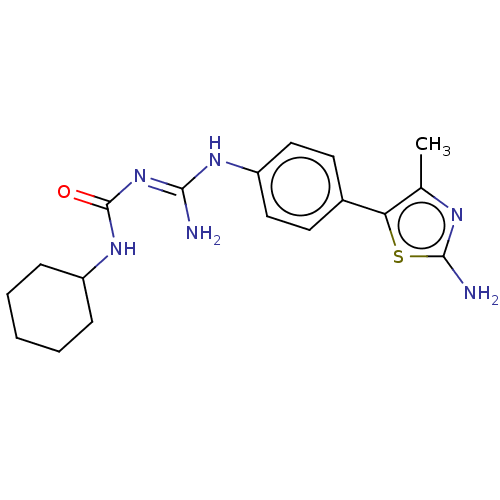
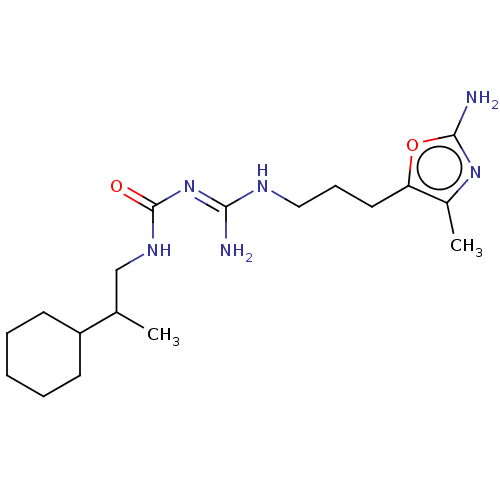
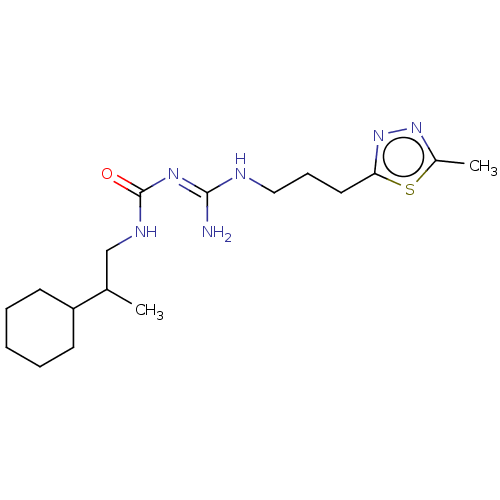
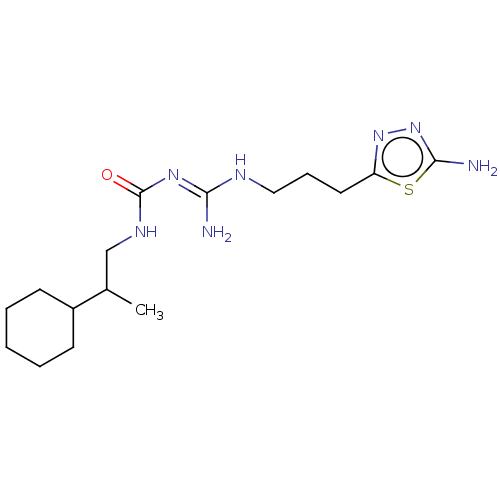
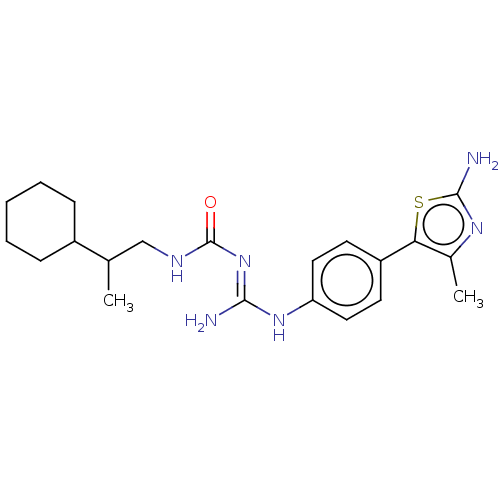
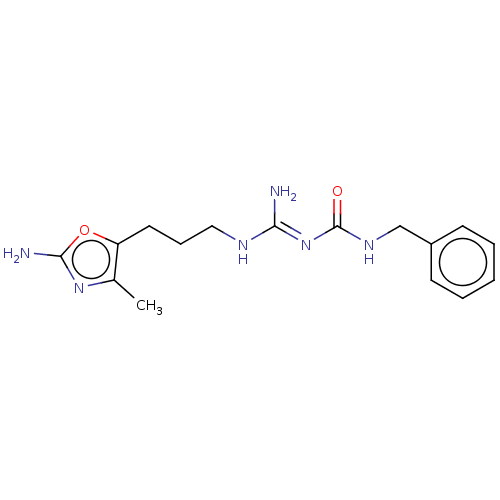
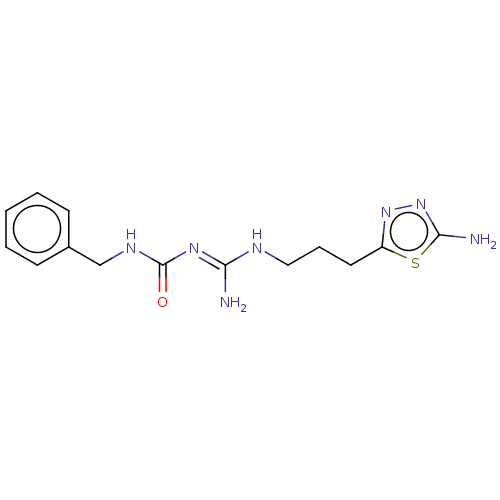
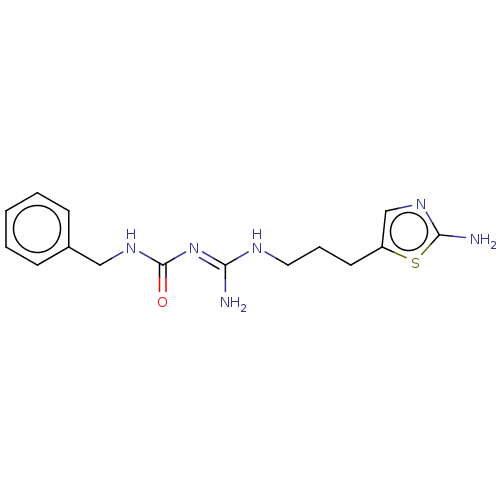
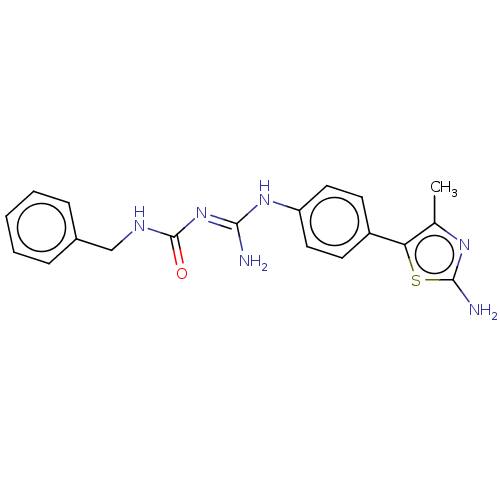
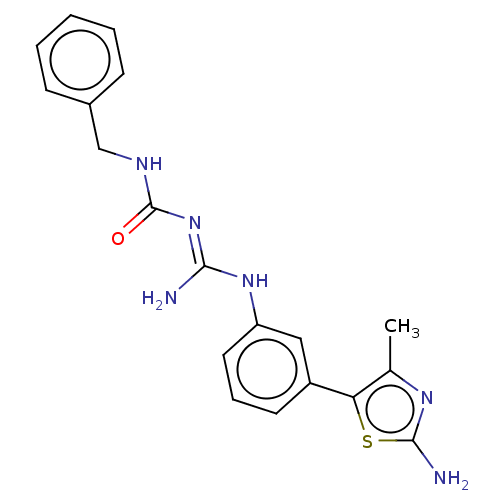
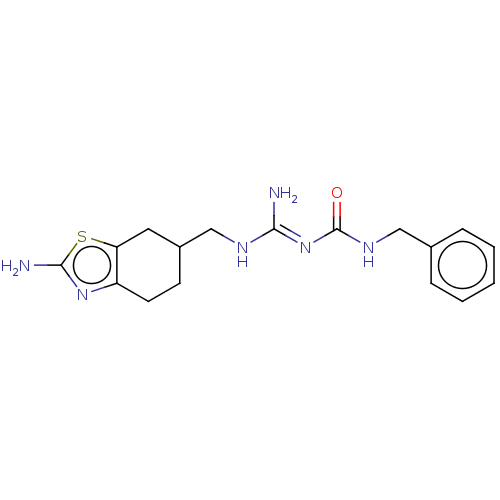
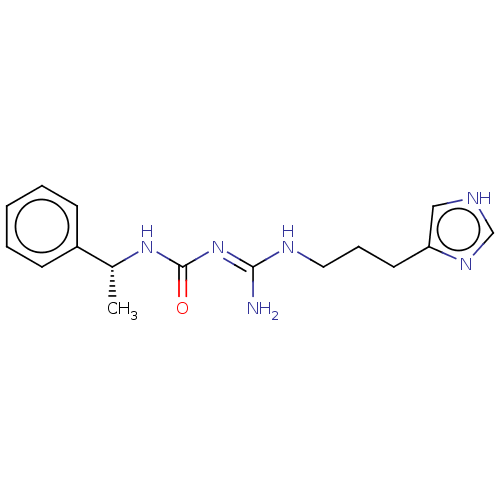
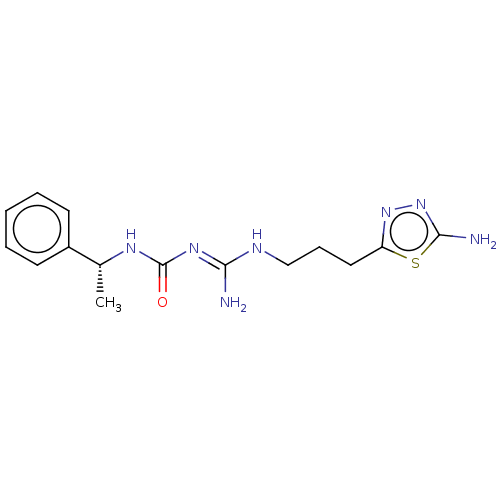
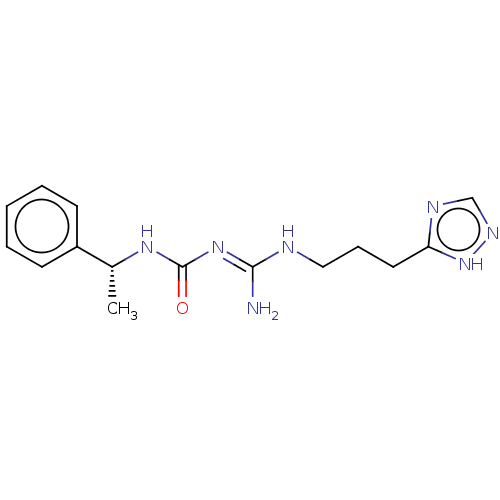
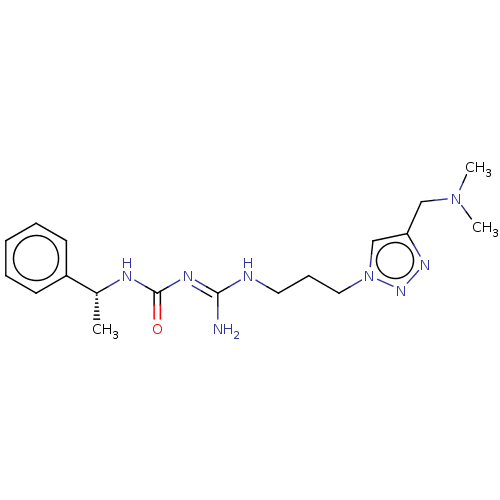
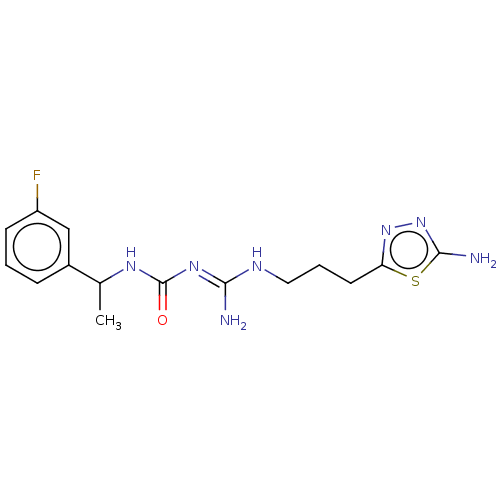
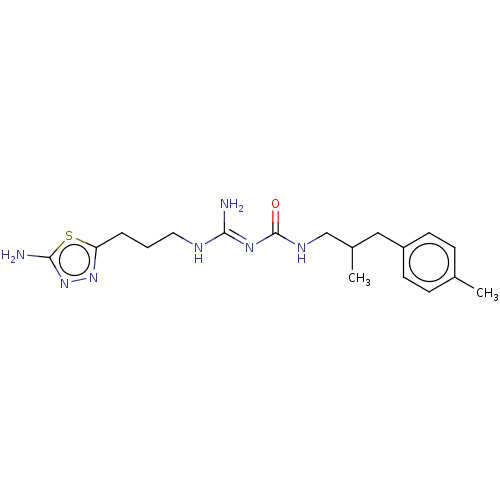
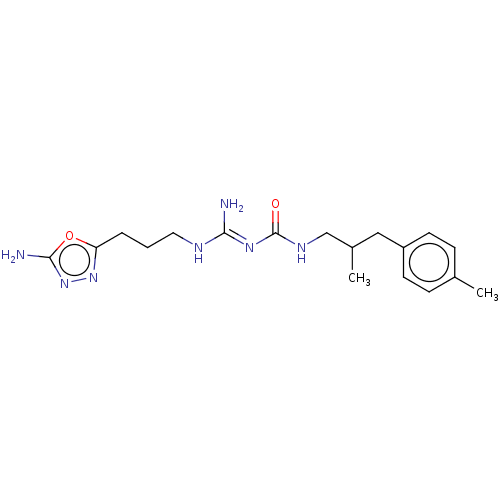
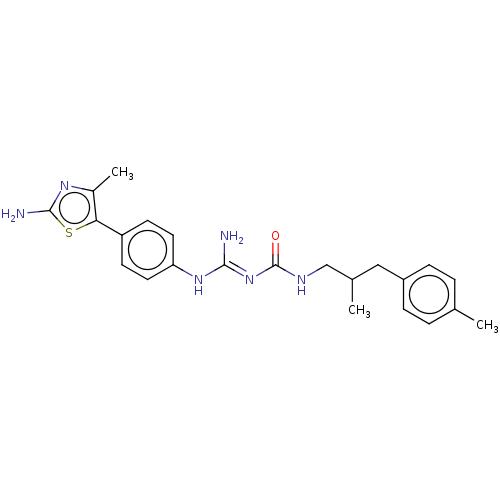
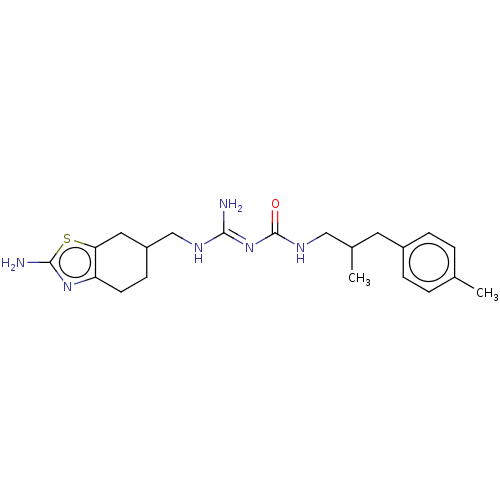
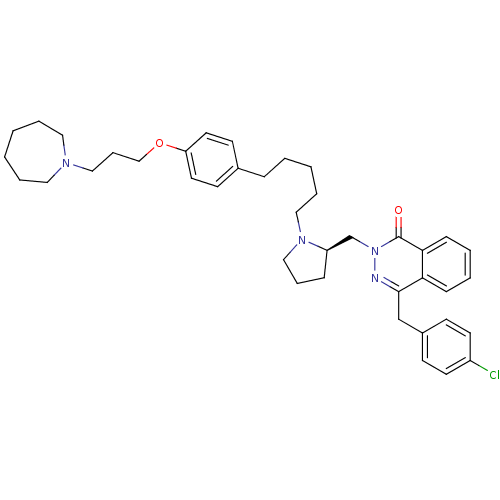
Affinity DataKd: 0.0870nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
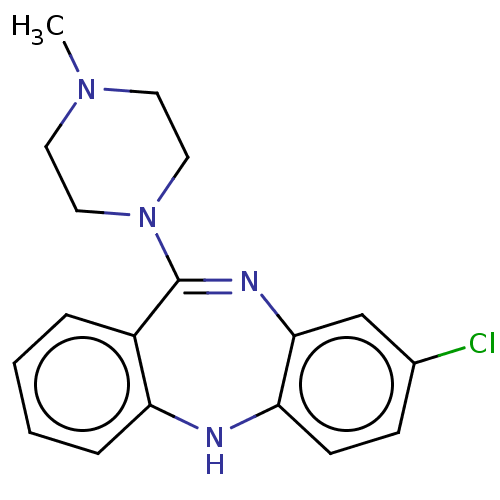
Affinity DataKd: 0.200nMAssay Description:Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal...More data for this Ligand-Target Pair
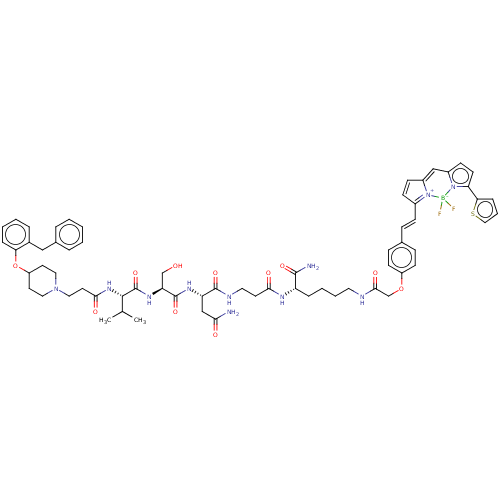
Affinity DataKd: 0.794nMAssay Description:Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal...More data for this Ligand-Target Pair
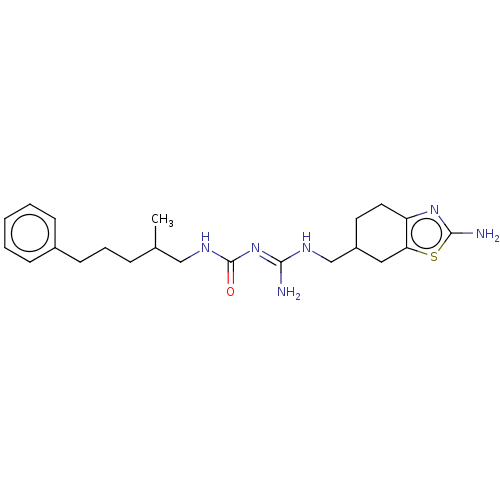
Affinity DataKd: 1.26nMAssay Description:Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal...More data for this Ligand-Target Pair
Affinity DataKd: 1.26nMAssay Description:Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal...More data for this Ligand-Target Pair
Affinity DataKd: 3.10nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKd: 3.98nMAssay Description:Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal...More data for this Ligand-Target Pair
Affinity DataKd: 4nMAssay Description:Binding affinity to NLuc-tagged human H1R expressed in HEK293T cells using NanoGlo as substrate preincubated for 2 hrs followed by substrate addition...More data for this Ligand-Target Pair
Affinity DataKd: 4nMAssay Description:Binding affinity to NLuc-tagged human H1R expressed in HEK293T cells using NanoGlo as substrate preincubated for 2 hrs followed by substrate addition...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair
Affinity DataKd: 4.5nMAssay Description:Displacement of [3H] mepyramine from human histamine H1 receptor stably expressed in baculovirus infected Sf9 cell membrane co-expressing RGS4 incuba...More data for this Ligand-Target Pair


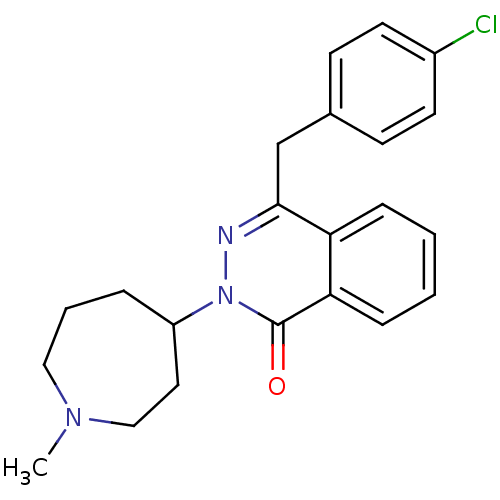





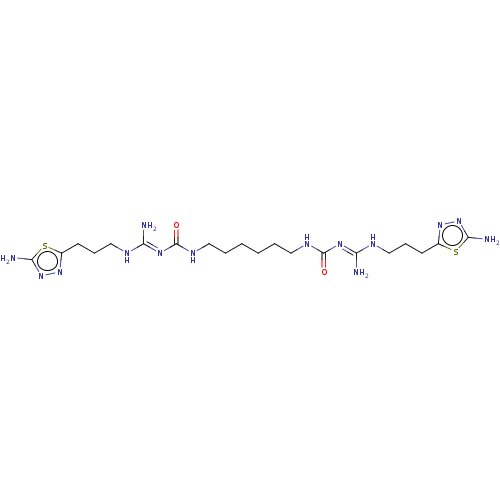
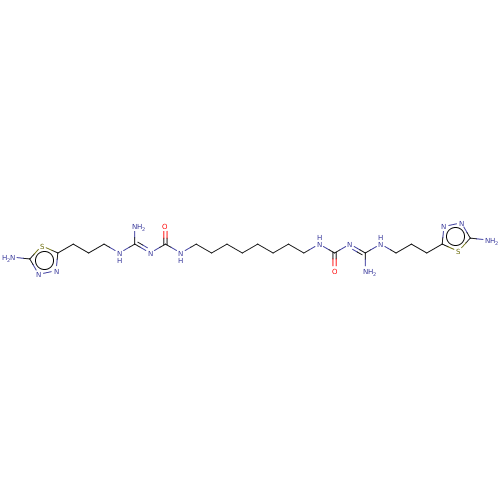
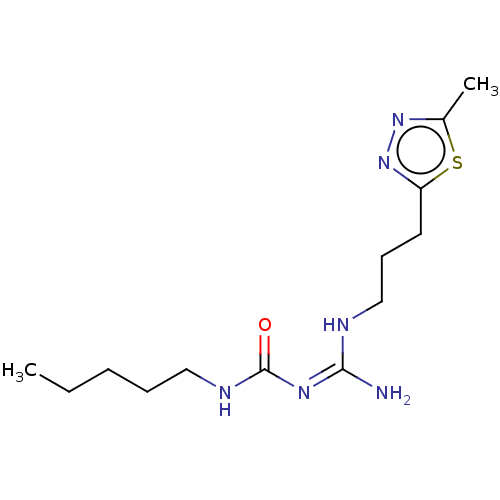
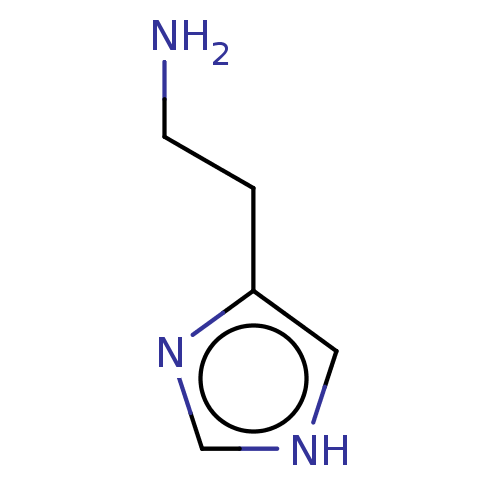
 3D Structure (crystal)
3D Structure (crystal)