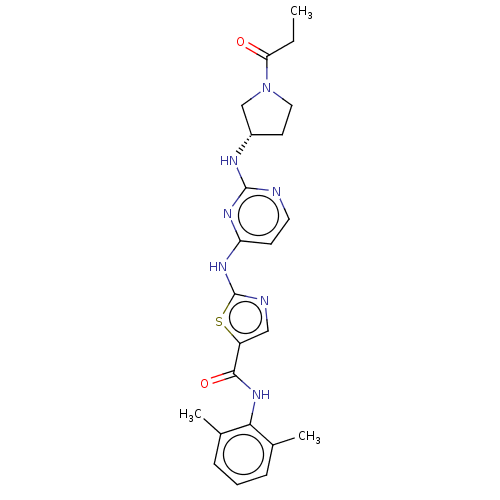
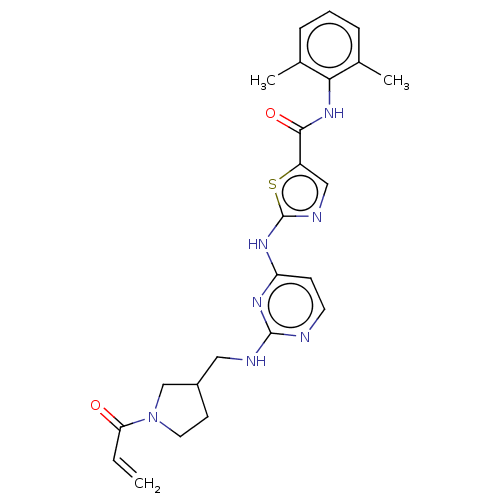
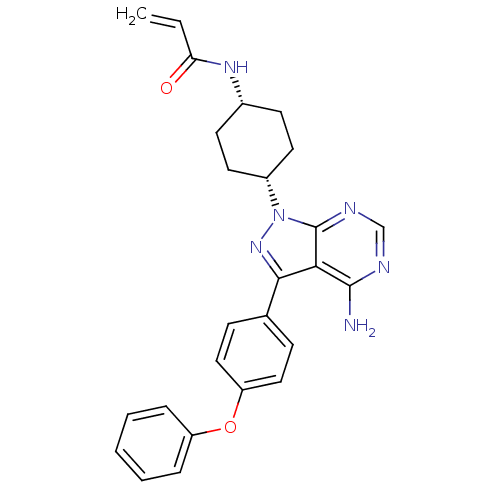
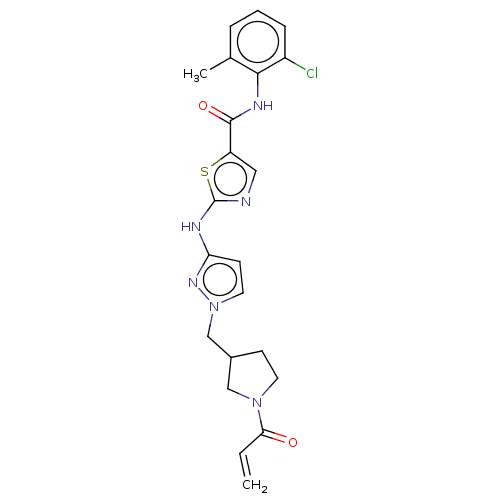
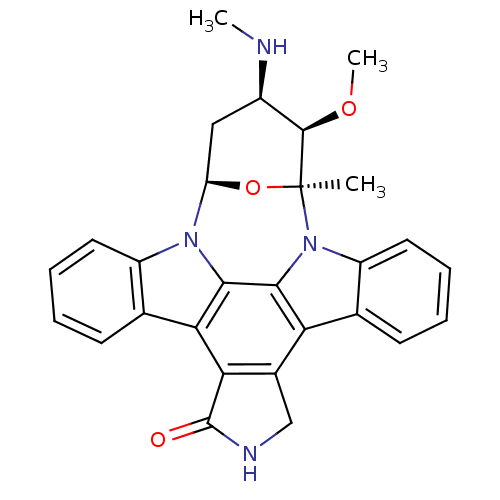
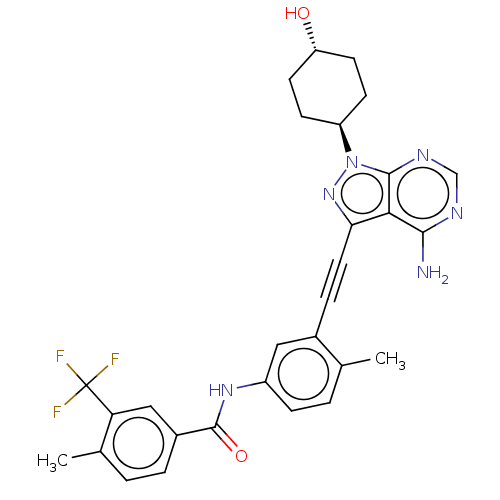
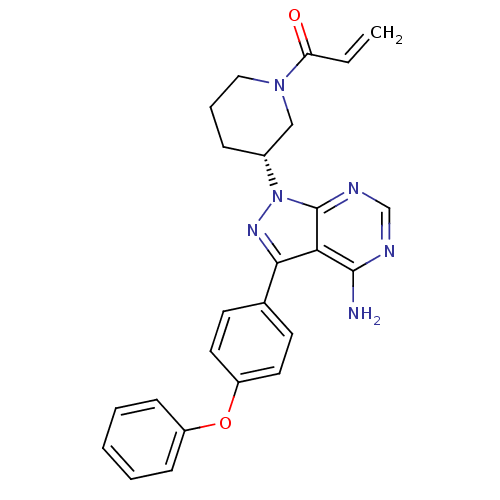
Affinity DataIC50: 0.110nMAssay Description:Binding affinity to human HCK using KVEKIGEGTYGVVYK as substrate by radiometric hotspot kinase assayMore data for this Ligand-Target Pair
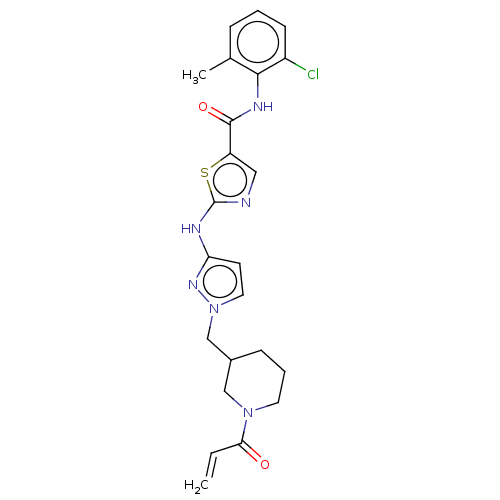
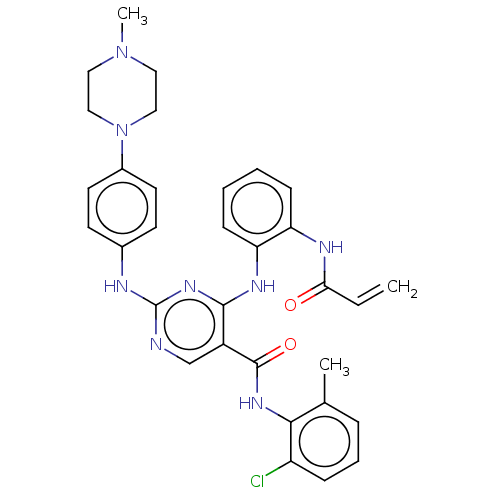
Affinity DataIC50: 0.257nMAssay Description:Inhibition of HCK (unknown origin)More data for this Ligand-Target Pair
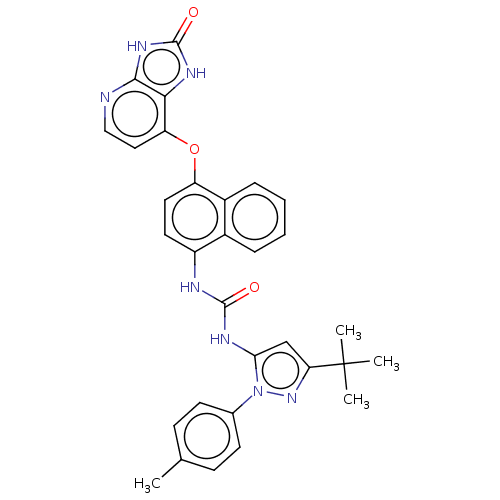
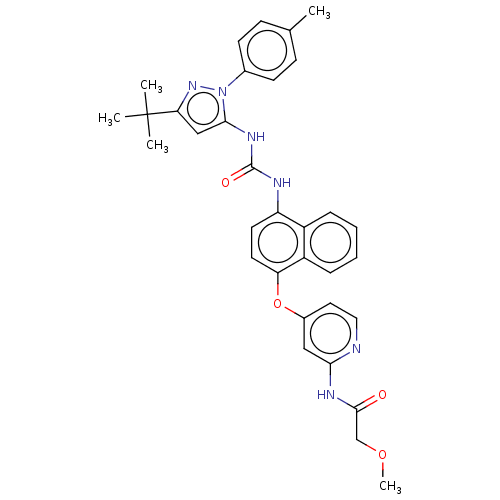
Affinity DataIC50: 0.260nMAssay Description:Inhibition of HCK (unknown origin)More data for this Ligand-Target Pair
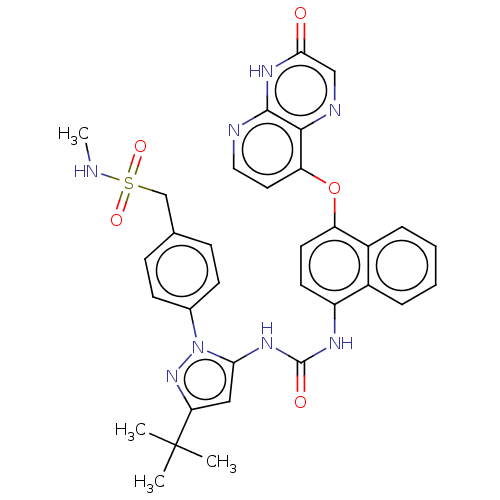
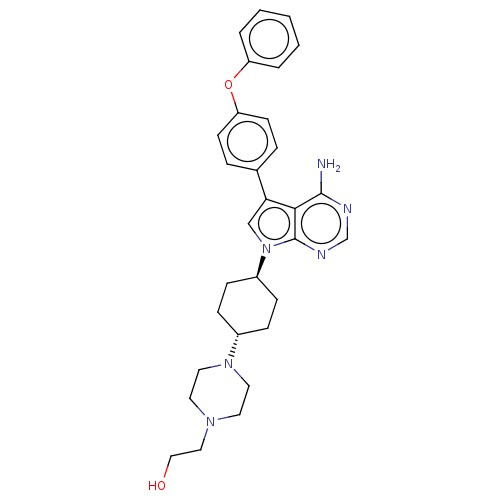
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.427nMAssay Description:Inhibition of HCK (75 to 526 residues) (unknown origin) expressed in Sf9 insect cells after 120 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inhibition of human HCK (81 to 526 residues) expressed in Sf9 insect cells after 120 minsMore data for this Ligand-Target Pair
Affinity DataIC50: <0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: <0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: <0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: <0.495nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: <0.5nMAssay Description:Inhibition of HCK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: <0.5nMpH: 7.5 T: 2°CAssay Description:Fluorescence assay used for determination of catch and release efficiency. More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 0.730nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
Affinity DataIC50: 1.64nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human HCK using KVEKIGEGTYGVVYK as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.94nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: <2nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: <2nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: <2nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: <2nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:inhibitory activity against Hck kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 2.42nMAssay Description:The inhibitory activities of exemplary compounds described herein against select protein kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 2.43nMAssay Description:The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of full-length human N-terminal GST-tagged HCK expressed in baculovirus expression system by Z'-LYTE assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human HCK (230 to 497 residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of HCK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human HCK using KVEKIGEGTYGVVYK as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of HCK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of HCK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of HCK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition...More data for this Ligand-Target Pair

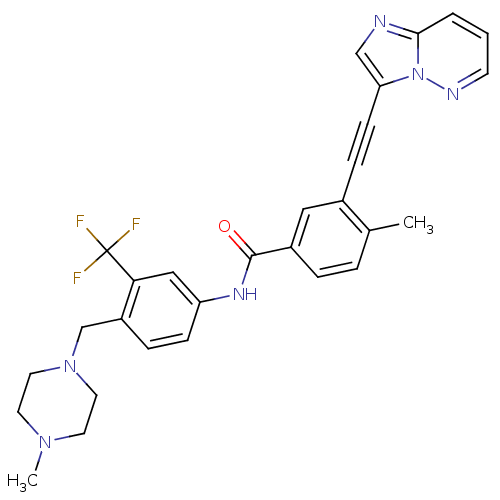


 3D Structure (crystal)
3D Structure (crystal)