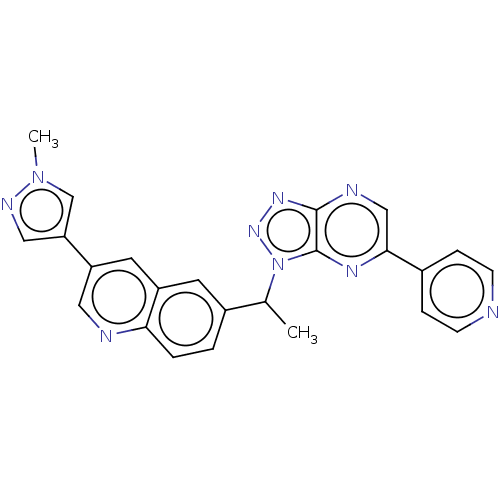
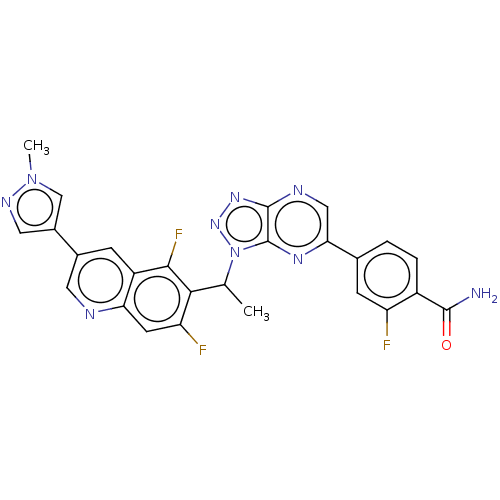
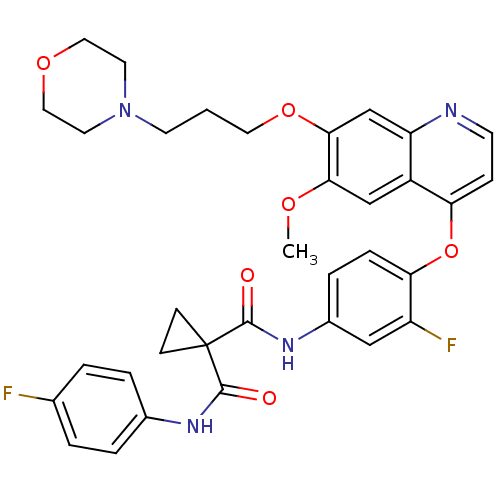
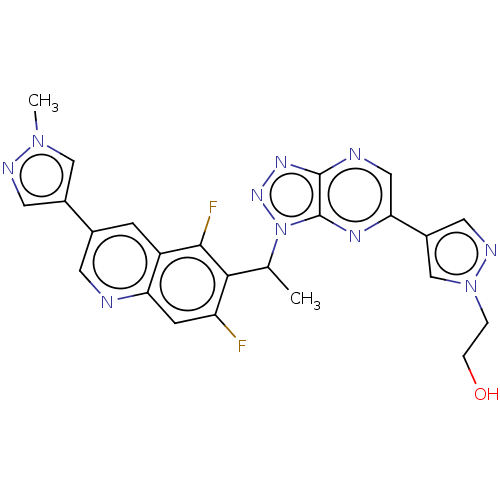
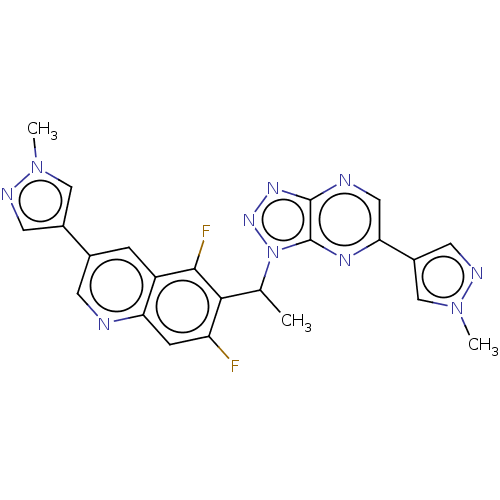
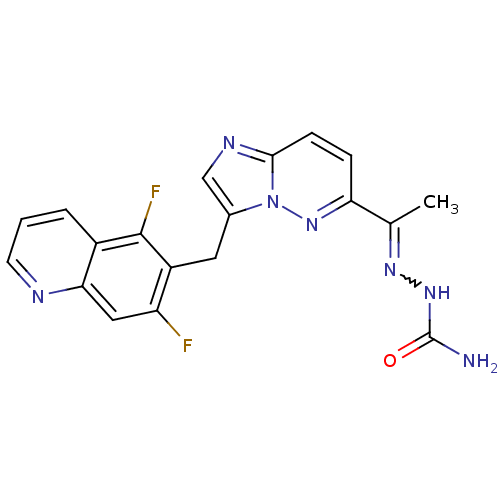
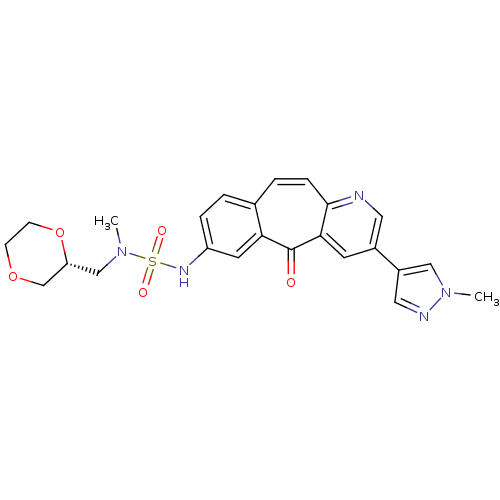
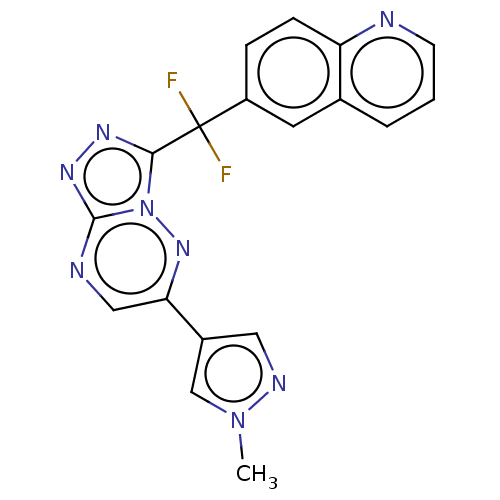
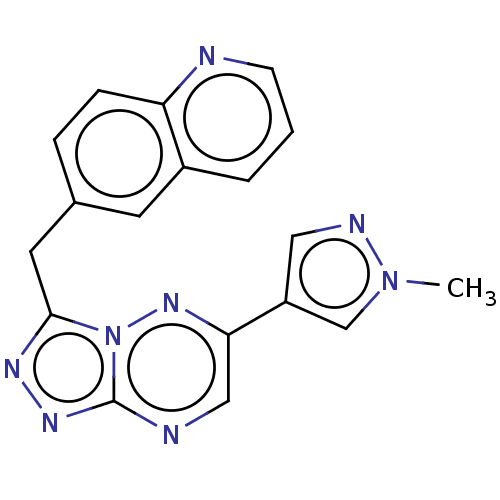
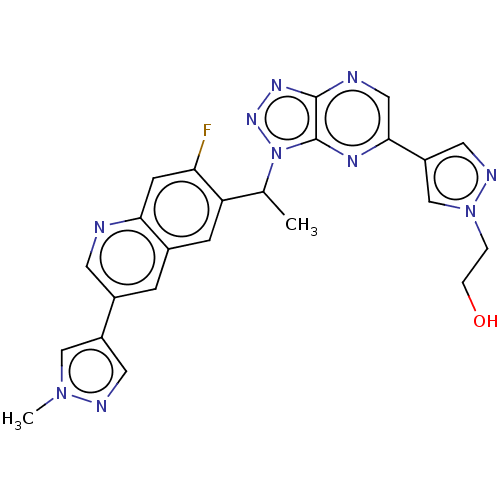
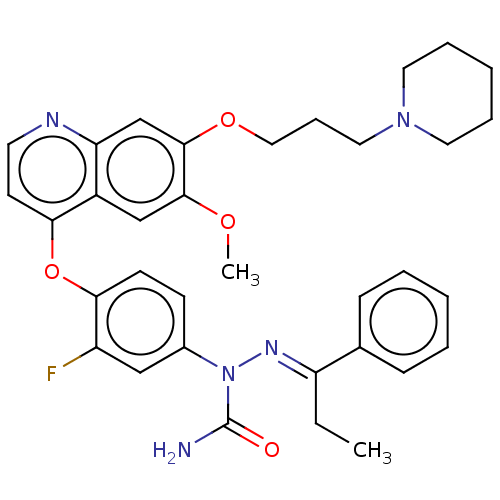
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.0190nMAssay Description:Inhibition of human recombinant c-MET in presence of ATP by HTRF assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.0350nMAssay Description:Inhibition of human recombinant c-MET in presence of ATP by HTRF assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Inhibition of c-Met (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Inhibition of C-Met (unknown origin) using polu (Glu,Tyr)4:1 substrate after 30 mins incubation by multi-well spectrophotometryMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human recombinant c-Met-catalyzed phosphorylation of N-biotinylated peptide (EQEDEPEGDYFEWLE-CONH2) by time-resolved fluorescence reson...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysisMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:c-Met Kinase Activity was Measured with an ELISA Reader. The Specific Operation as Follows:To the plate filled with 0.25 mg/mL PGT, the compounds, 50...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of c-MET (unknown origin) using TK substrate-biotin peptide as substrate preincubated for 5 to 10 mins followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of c-MET (unknown origin) using TK substrate-biotin peptide as substrate preincubated for 5 to 10 mins followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of c-MET (unknown origin) after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of cMET (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human full length MET by scintillation counting method in presence of 33P-gammaATPMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of NH2-terminal His-tagged MET kinase domain (1051 to 1348 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells p...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-MET (unknown origin) using TK substrate-biotin peptide as substrate preincubated for 5 to 10 mins followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-MET (unknown origin) using TK substrate-biotin peptide as substrate preincubated for 5 to 10 mins followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-MET (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met kinase (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of Met (unknown origin) using poly(Glu, Tyr) 4:1 as substrate preincubated for 60 mins followed by substrate addition measured after 2 to ...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate preincubated for 60 mins followed by substrate addition measured after 2 ...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met (unknown origin) using poly(Glu, Tyr)4:1 as substrate preincubated for 60 mins followed by substrate addition measured after 2 to...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.430nMAssay Description:Inhibition of c-Met in human EBC-1 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.440nMAssay Description:Inhibition of recombinant human His-tagged c-Met cytoplasmic domain (956 to 1390 residues) expressed in baculovirus expression system using poly (Glu...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.460nMAssay Description:Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.460nMAssay Description:Inhibition of c-Met in human MKN45 cells assessed as reduction in cell proliferation after 72 hrs by SRB or MTT assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.480nMAssay Description:Inhibition of c-MET (unknown origin) using TK substrate-biotin peptide as substrate preincubated for 5 to 10 mins followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.480nMAssay Description:The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.480nMAssay Description:Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.490nMAssay Description:Inhibition of recombinant c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate incubated for 60 mins by ELISAMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human c-METMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of c-Met autophosphorylation in human A549 cells after 1 hr by ELISAMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of GST-tagged c-Met (unknown origin) assessed as phosphotyrosine levels preincubated foe 15 mins followed by addition of poly(glutamic aci...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL
Key Laboratory Of Technology Of Drug Preparation (Zhengzhou University)
Curated by ChEMBL



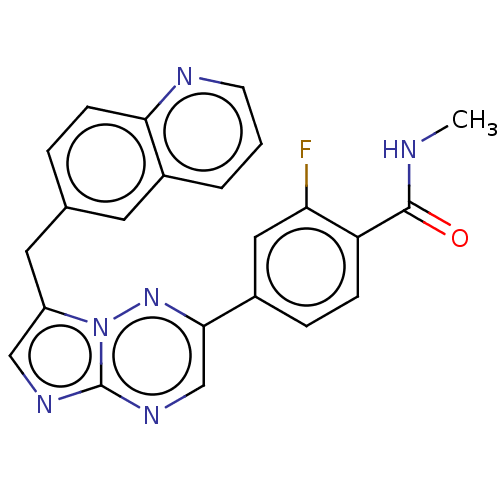
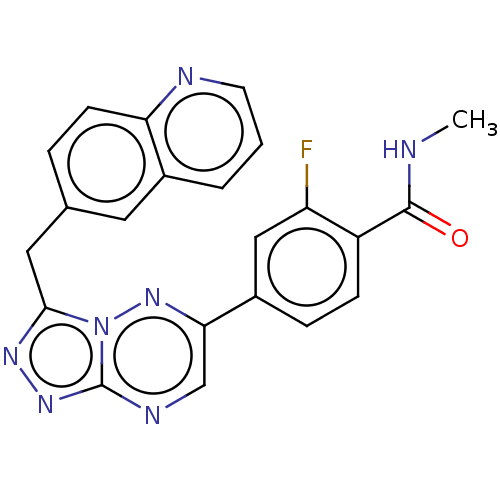







 3D Structure (crystal)
3D Structure (crystal)