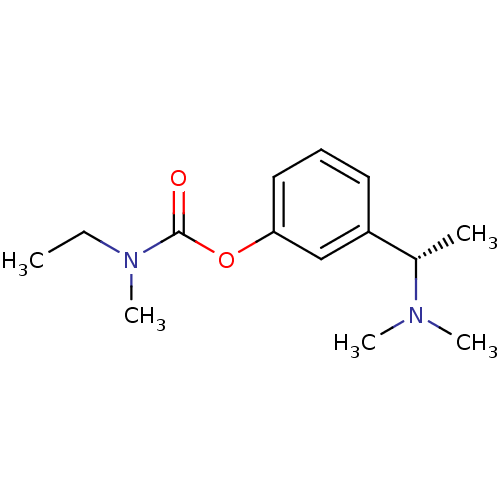
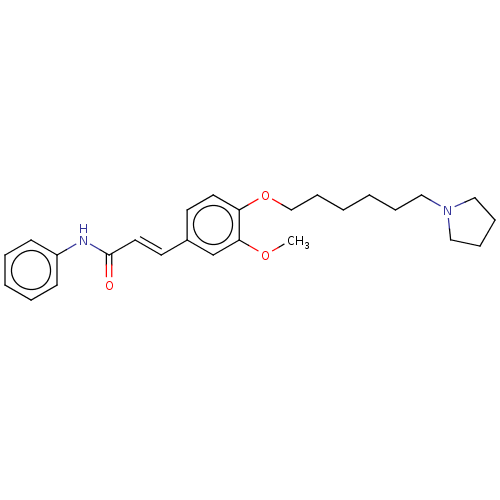
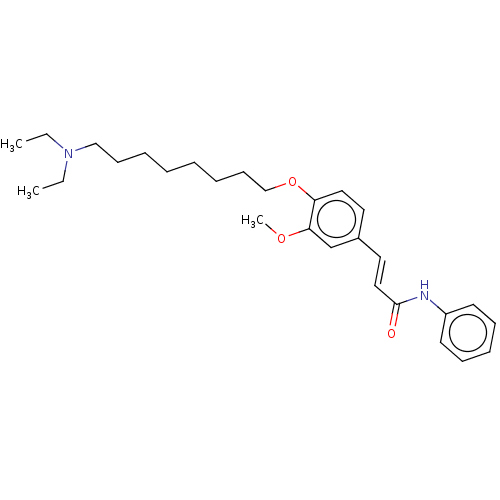
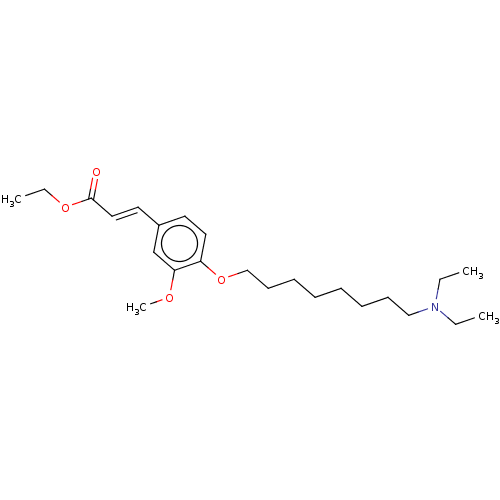
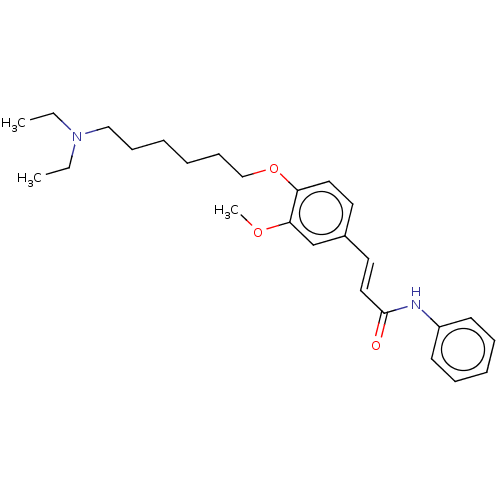
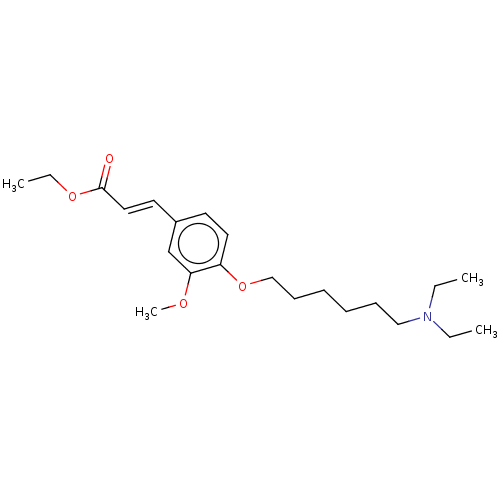
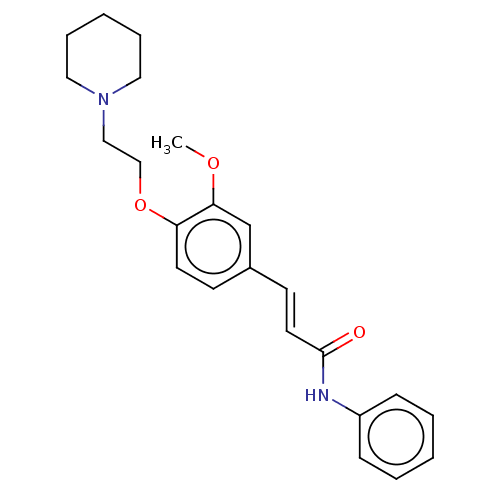
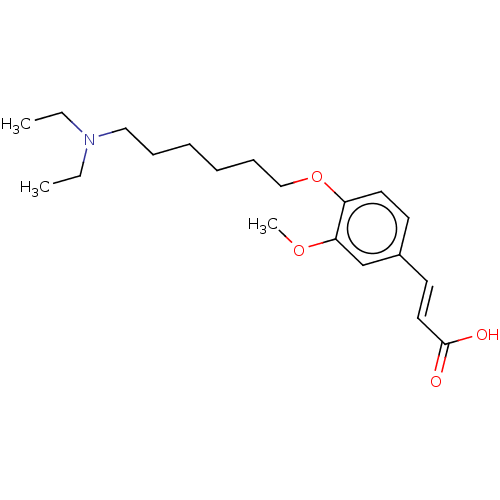
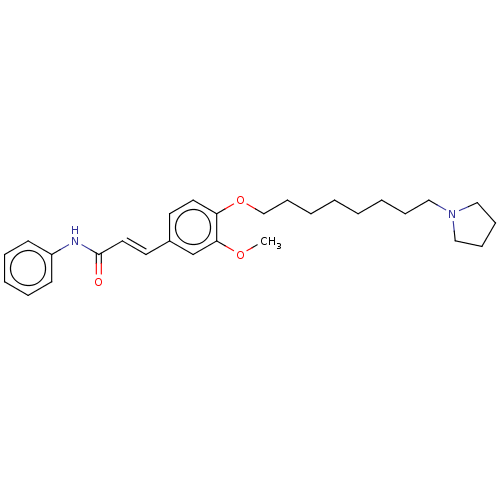
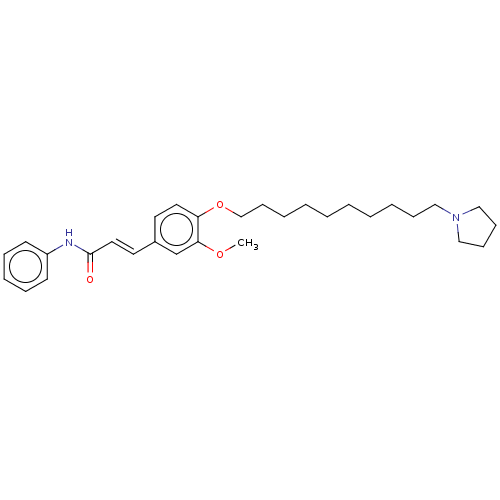
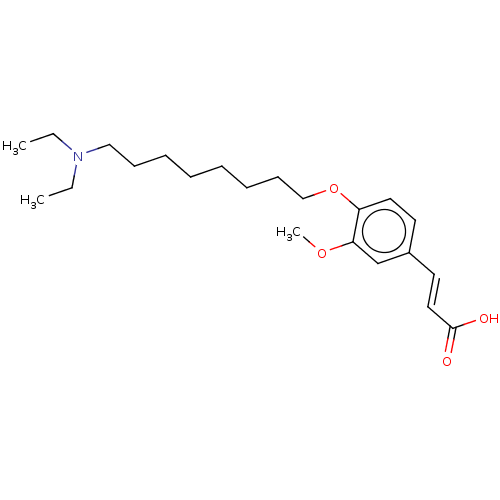
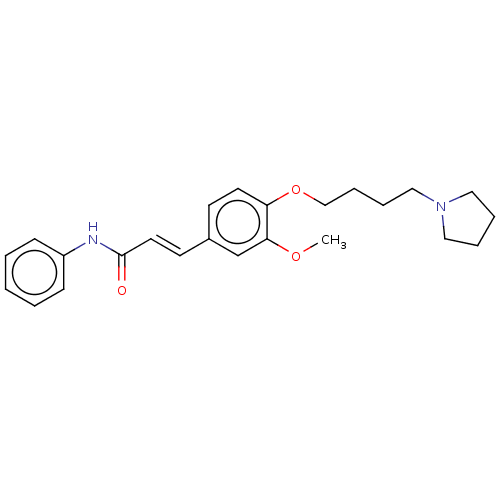
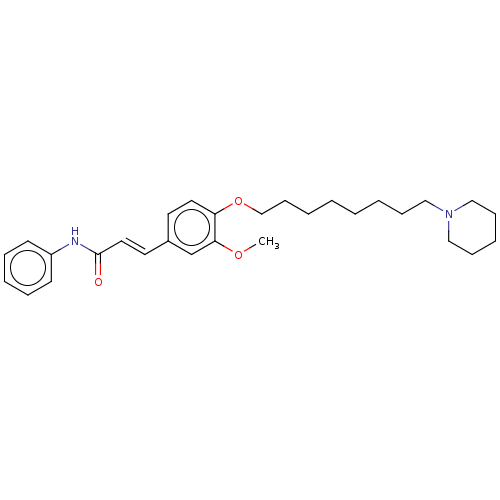
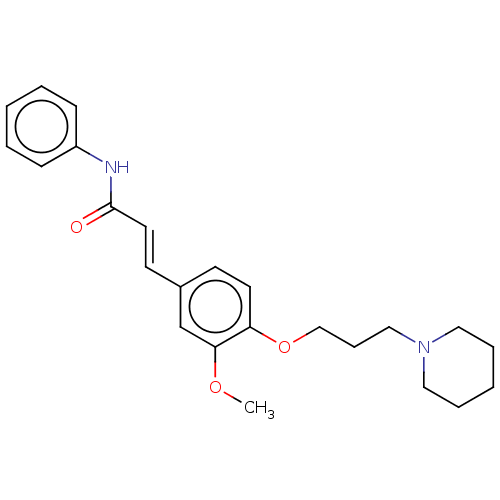
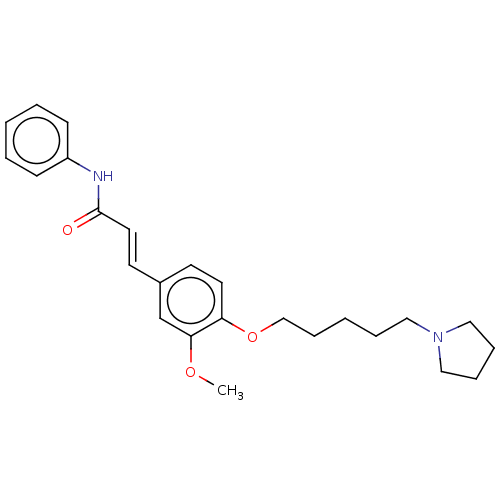
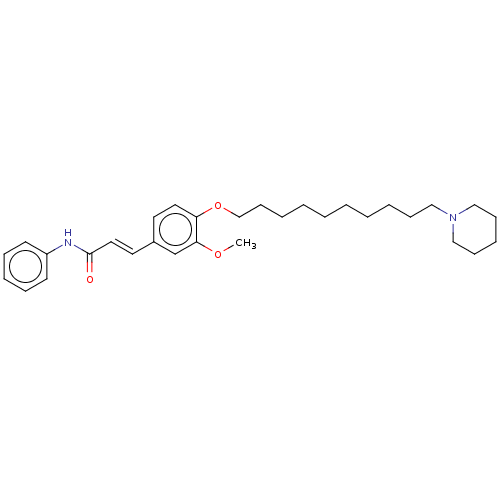
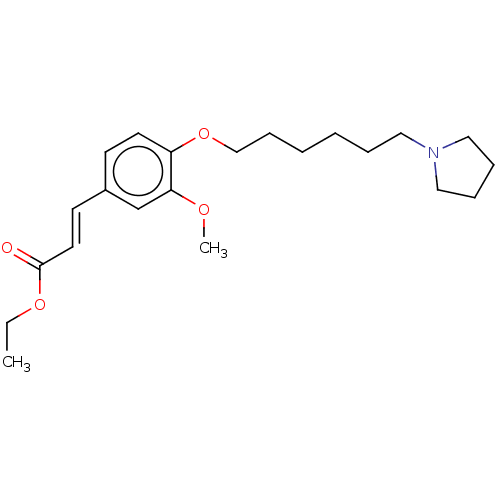
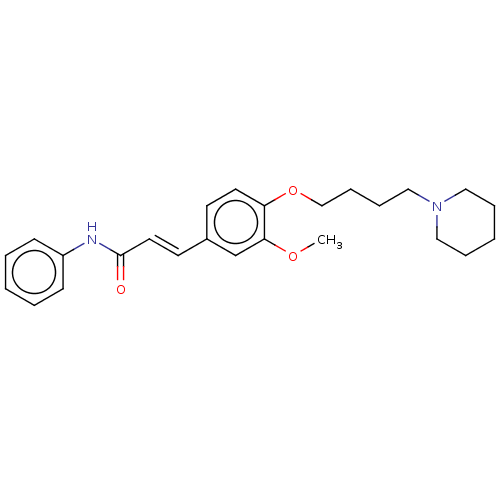
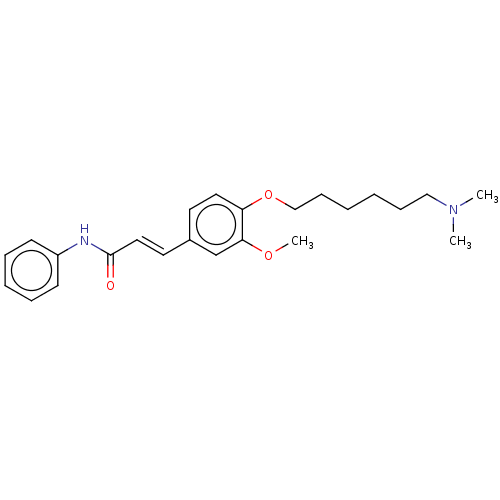
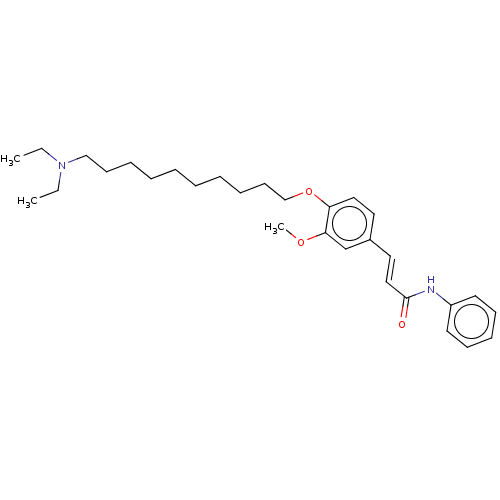
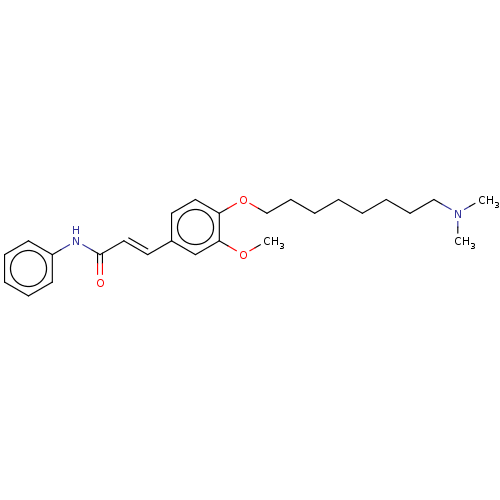
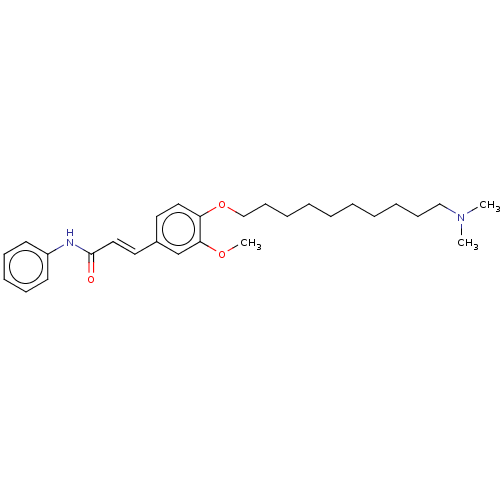
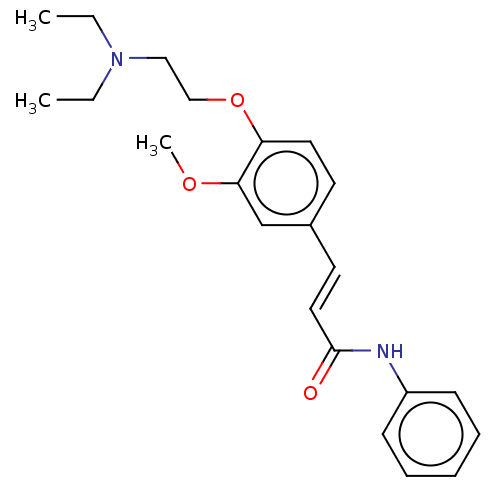
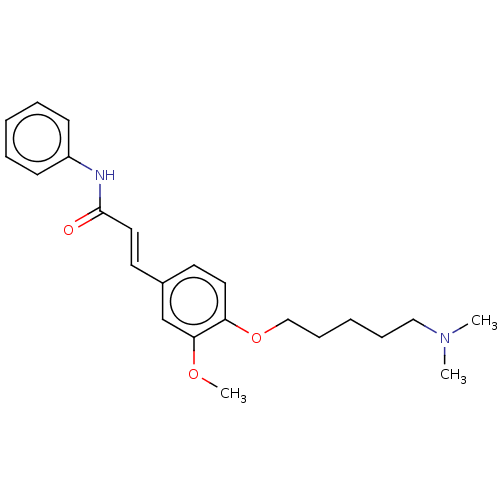
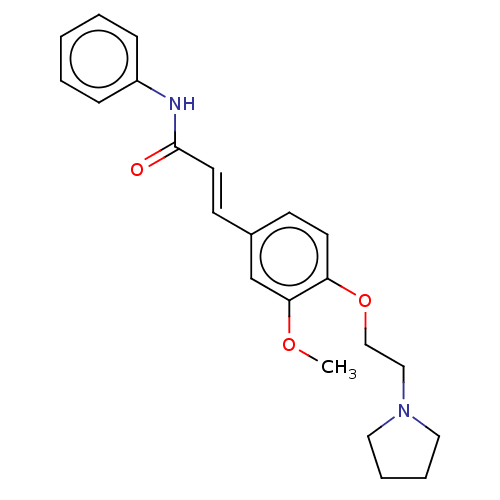
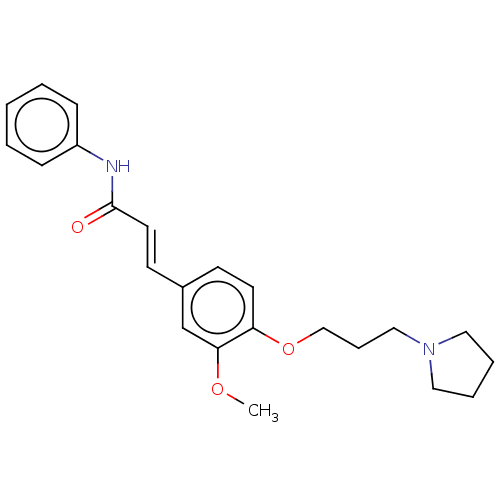
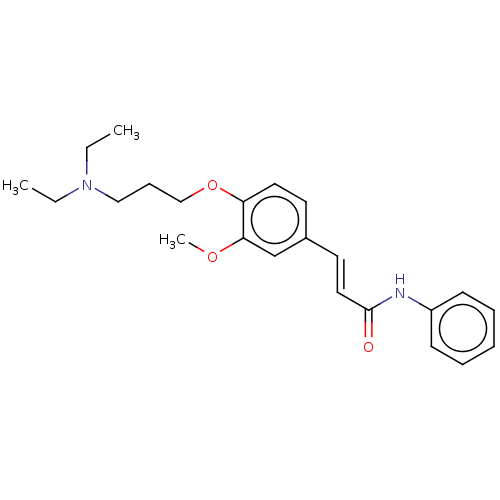
Affinity DataKi: 480nMAssay Description:Competitive inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 920nMAssay Description:Noncompetitive inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 710nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.11E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.94E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.23E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.34E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.65E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.67E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.91E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.99E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.18E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.83E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.03E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.12E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.78E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.78E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.84E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.86E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.05E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.45E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.69E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.21E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.72E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.14E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.47E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.95E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.93E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+4nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.11E+4nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.14E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+4nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.44E+4nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.49E+4nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.68E+4nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair